Summary
This article presents primary results from The Stabilization of Plaques Using Darapladib-TIMI 52 trial [SOLID-TIMI 52; NCT01000727], demonstrating that darapladib is ineffective at reducing major coronary events when started within 30 days after an acute coronary syndrome (ACS) event.
- Cardiology Clinical Trials
- Cardiology
- Coronary Artery Disease
- Cardiology Clinical Trials
- Coronary Artery Disease
Michelle O'Donoghue, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented primary results from The Stabilization of Plaques Using Darapladib-TIMI 52 trial [SOLID-TIMI 52; NCT01000727], demonstrating that darapladib is ineffective at reducing major coronary events when started within 30 days after an acute coronary syndrome (ACS) event.
By way of background, lipoprotein-associated phospholipase A2 (Lp-PLA2), a calcium-independent phospholipase A2 that circulates in plasma in association with low-density lipoprotein particles, has been hypothesized to play a causal role in the development of atherosclerosis and to contribute to plaque instability through pathways related to inflammation. Darapladib is an enteric-coated, direct Lp-PLA2 inhibitor that reduces enzyme activity in plasma and atherosclerotic plaques [Boekholdt SM et al. Circulation. 2008].
The SOLID-TIMI 52 trial was a double-blind, placebo-controlled, phase 3 study that was conducted at 868 sites in 36 countries to compare the safety and efficacy of darapladib with placebo for patients with ACSs if started within 30 days after hospitalization. It enrolled 13 026 participants within 30 days of hospitalization with ACSs. Qualifying events were most commonly STEMI (45.2%), NSTEMI (42.7%), and unstable angina (12.2%) and were balanced between the darapladib and placebo treatment arms.
Participants were randomized 1:1 to darapladib (160 mg once daily; n = 6504) or matching placebo (n = 6522), with a median follow-up duration of 2.5 years. The primary end point was the composite of coronary heart disease (CHD) death, myocardial infarction (MI), or urgent coronary revascularization for myocardial ischemia. Secondary end points included the composite of cardiovascular (CV) death, MI, or stroke; total coronary events; CHD death or MI; any coronary revascularization; individual components of the primary end point; and all-cause mortality.
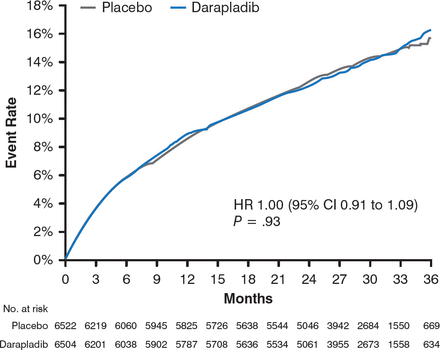
After 3 years, the results demonstrated no significant difference in the occurrence of the primary end point between participants in the darapladib and placebo arms (16.3% vs 15.6%; HR, 1.00; 95% CI, 0.91 to 1.09; P = .93; Figure 1).
Occurrence of the Primary End Point in the Darapladib and Placebo Treatment Arms
CHD, coronary heart disease; MI, myocardial infarction.
Reproduced with permission from M. O'Donoghue, MD.
Similarly, darapladib did not significantly reduce the risk for the study's secondary end points of CV death, MI, or stroke (15.0% vs 15.0%; HR, 0.99; 95% CI, 0.90 to 1.09; P = .78). Additionally, there were no significant differences between the treatment arms for the individual components of the primary end points, for additional secondary and exploratory end points, or all-cause mortality (7.3% vs 7.1%; HR, 0. 94; 95% CI, 0.82 to 1.08; P = .40).
Participants in the darapladib arm reported an odor-related complaint (predominantly of the urine, feces, and skin; 11.5% vs 2.5%) and diarrhea (10.6% vs 5.6%) more frequently than those in the placebo arm.
These data therefore do not support a strategy of targeted Lp-PLA2 inhibition with darapladib on a background of optimal medical therapy in patients stabilized after ACS events, concluded Dr O'Donoghue. However, ongoing trials are evaluating the clinical utility of other novel therapeutics that target alternative pathways of inflammation.
- © 2014 MD Conference Express®