Summary
Colchicine (COL) administered perioperatively in patients undergoing cardiac surgery reduced the incidence of the primary end point of postpericardiotomy syndrome (PPS) compared with placebo. Secondary end points of postoperative atrial fibrillation (POAF) and postoperative pleural and pericardial effusions were not significantly reduced in an investigator-initiated, double-blind, randomized clinical trial conducted at 11 centers in Italy. This article presents the results of the Colchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation trial [COPPS-2; Imazio M et al. JAMA. 2014].
- Cardiology
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
Colchicine (COL) administered perioperatively in patients undergoing cardiac surgery reduced the incidence of the primary end point of postpericardiotomy syndrome (PPS) compared with placebo. Secondary end points of postoperative atrial fibrillation (POAF) and postoperative pleural and pericardial effusions were not significantly reduced in an investigator-initiated, double-blind, randomized clinical trial conducted at 11 centers in Italy. Massimo Imazio, MD, University of Torino, Torino, Italy, presented the results of the Colchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation trial [COPPS-2; Imazio M et al. JAMA. 2014].
The COPPS-1 trial showed that COL started 3 days after cardiac surgery, compared with placebo, reduced the primary end point of PPS at 12 months (P = .002 vs placebo) and reduced secondary end points of POAF, disease-related hospitalizations, cardiac tamponade, constrictive pericarditis, and recurrent pericarditis [Imazio M et al. Eur Heart J 2010]. Because most POAF events occur in the first postoperative days (days 1 to 3), the authors supposed that an early administration of COL before surgery could improve its efficacy. The COPPS-2 trial was conducted to confirm the findings of COPPS-1 as well as determine whether oral COL might be even more effective at reducing PPS, POAF, and pleural and pericardial effusions if started prior to surgery.
Patients aged > 18 years undergoing cardiac surgery were randomized to placebo (n = 180) or COL (n = 180; 0.5 mg BID for patients ≥ 70 kg or 0.5 mg once daily for patients < 70 kg). COL was started 48 to 72 hours before surgery and continued until 1 month after surgery. Adherence to study medication was assessed by pill counts; unconscious patients were administered medication via a nasogastric tube. Follow-up continued for 3 months after surgery. Key exclusion criteria were current atrial fibrillation, being a candidate for cardiac transplantation, serum creatinine > 2.5 mg/dL, and a contraindication to COL.
The mean age of the patients was 67.5 years, and most were men (68.9%). Surgery was valvular alone in 36.4%, coronary artery bypass grafting alone in 33.9%, aortic surgery alone in 6.1%, and combinations of these in 23.6% of patients.
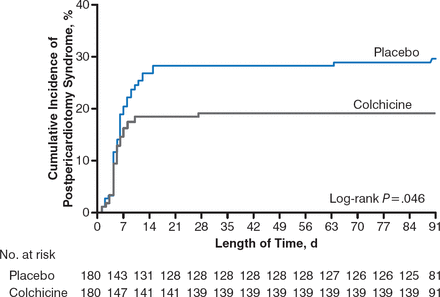
The primary end point of PPS occurred in 35 patients (19.4%) in the COL group and 53 patients (29.4%) in the placebo group (Figure 1). The absolute difference between the groups was 10% (95% CI, 1.1% to 18.7%), with a number needed to treat (NNT) of 10 to prevent 1 occurrence of PPS.
Kaplan-Meier Rate of PPS in Patients Receiving COL Versus Placebo
COL, colchicine; PPS, postpericardiotomy syndrome.
Reproduced with permission from M. Imazio, MD.
The secondary end point of POAF occurred in 33.9% of the COL group and 41.7% of the placebo group (absolute difference, 7.8%; 95% CI, −2.2% to 17.6%). Postoperative pericardial or pleural effusion occurred in 57.2% and 58.9% of the COL and placebo groups, respectively, in the intention-to-treat analysis.
Drug discontinuation was high at 21.7% and 17.8% of the COL and placebo groups, respectively. The difference between the groups was due largely to gastrointestinal intolerance (Table 1). Because of an expectation of significant discontinuation rates, a prespecified on-treatment analysis was performed, which demonstrated a reduction in POAF with COL compared with placebo (27% of 141 patients vs 41.2% of 148 patients, respectively); the absolute difference was 14.2% (95% CI, 3.3% to 24.7%) with an NNT of 7. It is likely that a lower drug discontinuation rate would have demonstrated a significant impact of COL on POAF in the intention-to-treat analysis.
Safety Profile of COL in the COPPS-2 Trial
In light of COL-induced gastrointestinal effects and strict trial exclusion criteria, careful consideration must be taken to select appropriate patients if COL is to be used as treatment for the prevention of PPS.
- © 2014 MD Conference Express®