Summary
Neuropathic pain is common in patients with diabetes but is not always treated. This article discusses treating patients with neuropathic pain by targeting pain, depression, and anxiety. In addition, she advised screening patients for cardiovascular autonomic neuropathy, teaching foot care, maintaining tight glycemic control, and investing in patient education.
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
Neuropathic pain is common in patients with diabetes but is not always treated. Pat Rafferty, PharmD, St. Louis College of Pharmacy, St. Louis, Missouri, USA, recommended aggressively treating patients with neuropathic pain by targeting pain, depression, and anxiety. In addition, she advised screening patients for cardiovascular autonomic neuropathy, teaching foot care, maintaining tight glycemic control, and investing in patient education.
Data from a 2011 English cohort of 15,962 patients with diabetes showed that 21% had painful diabetic peripheral neuropathy (PDPN) [Abbott CA et al. Diabetes Care 2011]. Another study in 350 diabetic patients with chronic PDPN reported that only 39% had never received treatment for pain [Daousi C et al. Diabet Med 2004]. Health care costs are 3 times higher for patients with PDPN than for matched controls [Smith HS, Argoff CE. Drugs 2011]. Diabetic neuropathies vary in their presentation and can be either diffused or focal. Dr. Rafferty focused her presentation on 2 diffused neuropathies: distal symmetric polyneuropathy, which accounts for 75% of all diabetic neuropathies, and cardiovascular autonomic neuropathy.
The American Diabetes Association guidelines suggest that all patients be screened for diabetic peripheral neuropathy and for the signs and symptoms of cardiovascular autonomic neuropathy. This screening should occur at the time of diagnosis in patients with type 2 diabetes and 5 years after diagnosis in patients with type 1 diabetes.
The symptoms of diabetic peripheral neuropathy are as follows:
-
Tingling
-
Pain—burning, shooting, aching
-
Evoked pain—allodynia, hyperesthesia
-
Unusual sensations—swelling, cold, walking on pebbles
They are often more severe at night and may also be described as numbness or a “dead” feeling. Symptoms occur symmetrically, in a “stocking and glove pattern.” The toes are affected first, and patients can lose their vibration and proprioceptive sensations, which can put them at risk for falls.
Diabetic peripheral neuropathy can be classified as peripheral or central. The causes of peripheral diabetic peripheral neuropathy are multifactorial and include changes in sodium, calcium, and potassium channel distribution and expression, altered neuropeptide expression, altered peripheral blood flow, axonal atrophy and degeneration, damage to small fibers, or increased glycemic flux. Central sensitization refers to the hyperexcitability of peripheral neurons with increasing input to the central nervous system.
There are many ways to assess pain severity in these patients. The visual analog scale and Brief Pain Inventory Short Form are very general and can assess any type of pain. The Neuropathic Pain Symptom Inventory, the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale, the painDETECT questionnaire, and the McGill Pain Questionnaire and McGill Neuropathic Pain Questionnaire are specific for neuropathic pain. Other useful assessment tools are the Norfolk Quality of Life Scale for assessing neuropathy-specific measures of quality of life. The Hospital Anxiety and Depression Scale and the Beck Depression Inventory are helpful for assessing depression.
Drugs used in the treatment of diabetic peripheral neuropathy target multiple pathways. Lidocaine and carbamazepine block nerve potentials. Similarly, ezogabine, already approved for partial seizures, is in early development for use in painful neuropathy. Gabapentin and pregabalin can block the release of neurotransmitters such as substance P that are directly related to the sensation of pain from voltage-gated calcium channels.
Diabetic peripheral neuropathy follows multiple pathogenic pathways, all of which lead to pain. In the polyol pathway, hyperglycemia activates an aldose reductase enzyme, leading to cell damage, nerve demyelination, and excess activation of inflammatory pathways via protein kinase C activation. Hyperglycemia is associated with an accumulation of advanced glycation end products in the neural tissue. Hyperglycemia ultimately leads to oxidative stress, nerve ischemia, and impaired nerve growth. Diabetic polyneuropathy damages myelinated and unmyelinated nerve fibers and can occlude the vasa nervorum.
Realistic expectations for the management of diabetic polyneuropathy include a 50% reduction in pain intensity and improvements in functional measures (quality of life, sleep, and mood), glycemic control, and possibly cardiovascular risk factors.
For patients with type 1 diabetes, there is high-quality evidence from the Diabetes Control and Complications Trial [DCCT; DCCT Research Group. N Engl J Med 1993], conducted from 1983 to 1993, showing that keeping blood glucose levels close to normal slows the onset and progression of diabetic retinopathy, nephropathy, and neuropathy.
The follow-up registry, the Epidemiology of Diabetes Interventions and Complications trial [EDIC; DCCT/EDIC Study Research Group. N Engl J Med 2005], reported a 42% reduced risk for any cardiovascular disease event and a 57% reduced risk for nonfatal heart attack, stroke, or death from cardiovascular disease more than 10 years after the end of the DCCT.
The Kumamoto study, conducted in nonobese patients with type 2 diabetes, reported that the cumulative percentages of worsening in retinopathy and nephropathy were significantly lower (p < .05) with intensive treatment compared with conventional therapy [Shichiri M et al. Diabetes Care 2000]. Similar findings were reported in the United Kingdom Prospective Diabetes Study and other trials in this patient population.
The only analgesics currently approved for diabetic polyneuropathy are duloxetine, pregabalin, and tapentadol. All other medications discussed are for offlabel or investigational use. According to Dr. Rafferty, Cochrane reviews have identified tricyclic antidepressants (TCAs), the serotonin-norepinephrine reuptake inhibitors (SNRIs) duloxetine and venlafaxine, the α-2-δ ligands gabapentin and pregabalin, opioids, tramadol, and capsaicin cream as the only treatments achieving at least 50% pain intensity reduction over baseline. Current guidelines recommend TCAs, α-2-δ ligands, and SNRIs as first-line therapy. Opioids are reserved for second- or third-line therapy. If first-line therapy drugs are inadequate, a rational approach should include switching to a medication with a different mechanism of action. When using additive therapies, SNRIs and selective serotonin reuptake inhibitors should be avoided in patients taking a TCA or tramadol because of the risk for serotonin syndrome. Topical therapies such as capsaicin, lidocaine, and amitriptyline may be useful. Topical nonsteroidal anti-inflammatory agents are not as effective for neuropathic pain as they are for arthritis pain. Comparative effectiveness trials have shown similar outcomes between duloxetine and amitriptyline [Kaur H et al. Diabetes Care 2011] and between duloxetine and pregabalin [COMBO-DN; Tesfaye S et al. Pain 2013].
Depression, anxiety, and pain are interrelated. According to Dr. Rafferty, 22% of patients with diabetes meet the diagnostic criteria for depression, while 32% meet the criteria for anxiety [Jain R et al. Curr Diab Rep 2011]. Diabetic patients who are also depressed have an increased risk for neuropathy (OR 1.94) compared with patients who are not depressed [Raval A et al. Indian J Med Res 2010]. Depression is associated with poor medication adherence in patients and poor compliance with exercise and dietary instructions.
Autonomic neuropathies can be difficult to diagnose. Diseases that mimic autonomic neuropathies include idiopathic orthostatic hypotension, multiple-system atrophy with autonomic failure, Addison's disease, hypopituitarism, hypovolemia, and thyroid disease. Use of certain medications (eg, anticholinergic agents, vasodilators, sympathetic blockers) may also result in symptoms similar to those seen with autonomic neuropathy.
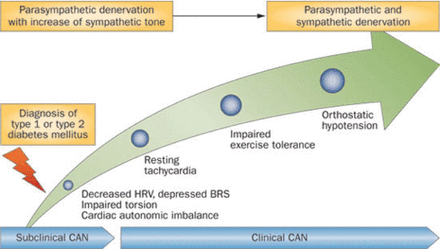
The clinical manifestations of cardiovascular autonomic neuropathy include exercise intolerance and orthostatic hypotension. Cardiovascular autonomic neuropathy is associated with silent myocardial infarction (RR, 1.96; 95% CI, 1.53 to 2.51; p < .001) and increased mortality (RR, 2.14; 95% CI, 1.83 to 2.51; p < .0001) [Vinik AI, Ziegler D. Circulation 2007]. Subclinical cardiovascular autonomic neuropathy, which is generally what is identified at diagnosis of type 1 or type 2 diabetes, can progress quickly to clinical, symptomatic cardiovascular autonomic neuropathy (Figure 1) [Kuehl M, Stevens MJ. Nat Rev Endocrinol 2012].
Progression of CAN Following the Diagnoses of Diabetes
BRS=baroreflex sensitivity; CAN=cardiovascular autonomic neuropathy; HRV=heart rate variability.
Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. Reproduced with permission from Nature Publishing Group.
Patients being tested for cardiovascular autonomic neuropathy should have assessment of heart rate response to Valsalva maneuver, deep breathing, and standing up and blood pressure response to standing up and sustained handgrip. These tests provide assessment of both parasympathetic and sympathetic responses. Ambulatory 24-hour blood pressure monitoring may also be useful.
Management of patients with cardiovascular autonomic neuropathy should include tight glycemic control. In addition, patients should be on statins, as these drugs may improve the functionality of the tissue within the vasculature. Evidence for the use of antioxidants and angiotensin-converting enzyme (ACE) inhibitors in the treatment of neuropathy is mixed. ACE inhibitors are very useful for hypertension and renal protection in diabetics. Beta-blockers may be used to reestablish parasympathetic-sympathetic balance. Aldose reductase inhibitors block the rate-limiting enzyme of the polyol pathway, thus decreasing the accumulation of sorbitol. In addition to pharmacological therapies, appropriate exercise and patient education are also necessary.
Drugs currently being evaluated for the treatment of diabetic neuropathy treatment include a new opioid receptor agonist, cebranopadol, and new endocannabinoid modulators. The use of vascular endothelial growth factor, stem cell transplantation, α-lipoic acid, and vitamin E (tocotrienols) for cardiovascular protection is currently being studied may be effective in this patient population.
- © 2014 MD Conference Express®