Summary
The main risk factors for hepatocellular carcinoma (HCC) are hepatitis C virus, hepatitis B virus, and alcohol consumption, which can lead to cirrhosis, genomic instability, and progression to HCC. This process involves a cycle of regeneration and necrosis that induces release of cytokines, proangiogenic factors, and profibrotic factors. Fibrosis and increased cell proliferation can result in formation of a dysplastic nodule, marked genomic instability, loss of p53, and development of HCC [Farazi PA, DePinho RA. Nat Rev Cancer. 2006].
- Gastrointestinal Cancers
- Gastrointestinal Cancers
The main risk factors for hepatocellular carcinoma (HCC) are hepatitis C virus, hepatitis B virus, and alcohol consumption, which can lead to cirrhosis, genomic instability, and progression to HCC. This process involves a cycle of regeneration and necrosis that induces release of cytokines, proangiogenic factors, and profibrotic factors. Fibrosis and increased cell proliferation can result in formation of a dysplastic nodule, marked genomic instability, loss of p53, and development of HCC [Farazi PA, DePinho RA. Nat Rev Cancer. 2006].
TARGETING MOLECULAR PATHWAYS IN HCC
Josep M. Llovet, MD, Mount Sinai School of Medicine, New York, New York, USA, discussed molecular pathways and targeted therapies for HCC. TERT promoter mutations are the earliest identified alterations in dysplastic liver nodules (25%) and the most frequent mutations in HCC (60%) [Nault JC et al. Nat Commun. 2013]. Other HCC mutations include CTNNB1 (24%), p53 (27%), Axin (8%), RAS (∼ 5%), PI3K (3%), ARID1a (12%), ARID1b (5.6%), and RPS6KA3 (9%) [Guichard C et al. Nat Gen. 2012].
The most common high-level amplifications are 6p21 (vascular endothelial growth factor) and 11q13 (cyclin D1 and fibroblast growth factor 19 [FGF19]). Seven percent of patients have high amplification of vascular endothelial growth factor A [Chiang DY et al Cancer Res. 2008], which activates hepatocyte growth factor secretion [Horwitz E et al. Cancer Discov. 2014]. In a retrospective study, patients with this amplification had markedly improved survival when treated with sorafenib [Horwitz E et al. Cancer Discov. 2014]. High-level FGF19 and CCND1 amplifications occur in HCC; tumorigenicity of HCC cells with the 11q13 amplicon is inhibited by blocking FGF19 or CCND1 [Sawey ET et al. Cancer Cell. 2011].
Other signaling pathways implicated in the development of HCC may provide potential targets for molecular therapies (Table 1).
Signaling Pathways Implicated in the Development of HCC
Sorafenib—an oral multikinase inhibitor of the vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf—has been the standard of care for HCC since the 2008 pivotal SHARP trial [Llovet JM et al. N Engl J Med. 2008]. Molecular therapies for HCC have been tested in > 300 Phase 2/3 trials, 56 of which are ongoing. Among 2014 Phase 3 trials, 11 of 18 have been stopped or had inconclusive or negative results.
Several Phase 1 and 2 studies are testing novel therapies against specific targets in HCC. Tivantinib, a MET inhibitor, causes cell death with or without MET amplification, acting on microtubule dynamics independently of MET [Michieli P, Di Nicolantonio F. Nat Rev Clin Oncol. 2013]. A Phase 3 study is investigating tivantinib for second-line therapy in patients with MET-positive HCC [NCT01755767].
CHOOSING THE OPTIMAL TREATMENT FOR HCC
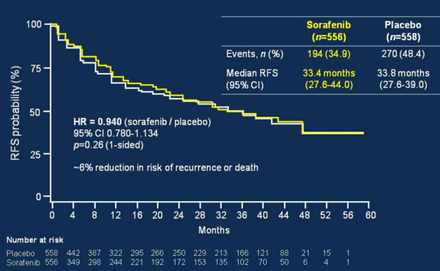
Chris Verslype, MD, PhD, University Hospital Leuven, Leuven, Belgium, discussed HCC treatment options for patients on the liver transplant waiting list. The Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma trial [STORM; NCT00692770] tested sorafenib versus placebo in patients with no residual disease following resection or ablation. The primary end point of relapse-free survival was not met, with median survival of 33.4 months in the sorafenib group versus 33.8 months in the placebo group (HR, 0.940; 95% CI, 0.780 to 1.134; p = .26; Figure 1) [Bruix J et al. J Clin Oncol. 2014 (suppl; abstr 4006)]. Sorafenib also did not improve overall survival (OS).
Sorafenib Versus Placebo: Relapse-Free Survival
Source: Bruix J et al. J Clin Oncol 2014 (abstr 4006).
Without therapy, patients with HCC on the transplant waiting list have a < 20% probability of dropping out at 6 months and < 40% at 12 months [Llovet JM et al. Hepatology. 1999]. There have been no randomized controlled trials of optimal bridging treatments for waiting list patients. The European Association for the Study of the Liver—European Organisation for Research and Treatment of Cancer clinical practice guidelines recommend treatment with local ablation or chemoembolization when waiting times are estimated to exceed 6 months [Llovet J et al. J Hepatol. 2012].
The evidence on radiofrequency ablation and transcatheter arterial chemoembolization (TACE) as bridging therapies is based on several studies. Survival after transplant is good in most radiofrequency ablation studies, but the intention-to-treat survival is more variable. The results of the TACE studies are also quite variable.
After bridging therapy, it is necessary to measure the response and monitor complications. Pretransplant imaging is correct in only 57% of patients, with understaging in 38% and overstaging in 5% [Galal A et al. HBP Dis Int. 2013]. In patients with complete necrosis of the original tumor, 3-year OS and disease-free survival (DFS) are both 100%; for partial necrosis, OS is 78% and DFS is 75% [El-Gazzaz G et al. Hepatobiliary Pancreat Dis Int. 2013]. Vandecaveye et al [Radiology. 2014] evaluated the relationship between response assessment and progressive-free survival (PFS) in patients treated with TACE. They found that the apparent diffusion coefficient ratio was a significant independent predictor of PFS (p < .001), with 93.3% accuracy.
According to Prof Verslype, HCC recurrence following locoregional treatment is the rule, and sorafenib does not prevent this. Little is known about the most appropriate management of patients waiting for transplantation, despite the availability of many therapies. The success of bridging on intention-to-treat survival depends on the tumor biology and response to therapy. Current unmet needs for management of patients on the waiting list include tools to assess tumor biology and early assessment of a maintained response to therapy.
NEW MOLECULAR TARGETED AGENTS
Andrew X. Zhu, MD, PhD, Harvard Medical School, Boston, Massachusetts, USA, overviewed current treatments and new molecular targeted agents for HCC. Sorafenib is the only systemic agent approved for the treatment of HCC, but it has modest efficacy in advanced HCC with Child A cirrhosis. Although there are no validated biomarkers for sorafenib in HCC, a few potential biomarkers have emerged (Table 2).
Potential Biomarkers of Sorafenib Efficacy in HCC
Several Phase 3 trials in advanced HCC have failed to demonstrate any benefit of treatment with sunitinib [Cheng AL et al. J Clin Oncol. 2013], brivanib [Johnson PJ et al. J Clin Oncol. 2013; Llovet JM et al. J Clin Oncol. 2013], linifanib [Cainap C et al. ASCO GI Symposium. 2012 (abstr)], erlotinib [Zhu A et al. ESMO 2012 (abstr)], or everolimus [Zhu A et al. JAMA. 2014].
A number of novel targeted therapies for HCC are currently undergoing testing in Phase 1 and 2 trials (Table 3).
Novel Targeted Therapies for HCC
The presenters in this session reviewed the treatment strategies and approaches for the treatment of HCC that have been and are currently under investigation. Thus far, sorafenib is the only systemic agent approved for HCC. Dr Zhu concluded that other novel agents with unique mechanisms of action should be explored. Identifying predictive markers and applying molecular classification are important for predicting response and enriching the population in future HCC trials.
- © 2014 MD Conference Express®