Summary
This article presents the results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation trial [PROTECT AF; NCT00129545] that compared the Watchman left atrial appendage device with warfarin therapy for patients with atrial fibrillation.
- Interventional Techniques & Devices
- Arrhythmias
- Cardiology
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Arrhythmias
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
Vivek Y. Reddy, MD, Mount Sinai Hospital, New York, New York, USA, presented the results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation trial [PROTECT AF; NCT00129545] that compared the Watchman left atrial appendage (LAA) device with warfarin therapy for patients with atrial fibrillation (AF).
The PROTECT AF trial randomly assigned 707 patients with AF to Watchman implantation (n=463) or warfarin (n=244). The primary efficacy end point was the composite of stroke, systemic embolism, or cardiovascular death. Patients treated with the Watchman device were also treated with warfarin and aspirin for 6 weeks after implant, clopidogrel and aspirin from 6 weeks to 6 months, and aspirin alone after 6 months. Analyses were performed at 600 patient-years and every 150 patient-years thereafter until 1500 patient-years.
After 3.8 years of follow-up, the primary efficacy event rates were 2.3 per 100 patient-years in the Watchman group versus 3.8 in the control group (rate ratio [RR], 0.60; 95% CI, 0.41 to 1.05; noninferiority p>0.999; superiority p=0.960) [Reddy VY et al. HRS 2013 (abstr LBA01–03)]. Table 1 shows the results for the components of the primary efficacy endpoint.
PROTECT AF: Components of the Primary Efficacy End Point
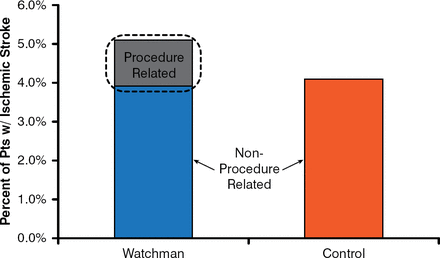
The stroke rates were 1.5 per 100 patient-years in the Watchman group versus 2.2 in the control group (RR, 0.68; 95% CI, 0.42 to 1.37; noninferiority p=0.999; superiority p=0.825). Ischemic strokes were increased in the Watchman group (1.4/patient-year) versus the control group (1.1/patient-year). It appears that the excess strokes were largely procedure related (Figure 1)—thereby confirming that LAA closure has the same benefit in preventing ischemic strokes as systemic oral anticoagulation.
Left Atrial Appendage Closure: Mechanism of Effect
Reproduced with permission from VY Reddy, MD.
The impact of stroke as measured by Modified Rankin Scores (MRS) was MRS 1.9 in the Watchman group versus 3.6 in the control group (p=0.031). Disabling strokes occurred at 0.5 per 100 patient-years in the Watchman group versus 1.2 per 100 patient-years in the control group.
In a prespecified analysis that analyzed the per-protocol population (which included device patients who stopped warfarin), results were 1.8 per 100 patient-years in the Watchman group versus 3.7 in the control group (RR, 0.50; 95% CI, 0.34 to 0.91; noninferiority p>0.999; superiority p=0.990). The rate of events in the post-hoc cohort of patients from the late-therapy analysis (including device patients following the discontinuation of clopidogrel) was 1.8 per 100 patient-years in the Watchman group versus 3.7 in the control group (RR, 0.50; 95% CI, 0.32 to 0.94; noninferiority p>0.999; superiority p=0.985). Intention-to-treat all-cause mortality was significantly lower in the Watchman group versus the control group (HR, 0.66; 95% CI, 0.45 to 0.98; p=0.0379).
The PREVAIL [NCT01182441] trial missed one of two efficacy end points, but it had a small number of events. The CAP registry data confirmed the PROTECT AF data demonstrating the superiority of the Watchman to warfarin. Safety event rates in all three trials were 9.9% in the first half and 4.8% in the second half of PROTECT AF, 4.1% in CAP, and 4.2% in PREVAIL.
Dr. Reddy concluded that interventional therapies for the prevention of stroke for patients with atrial fibrillation such as LAA closure are feasible and may become alternatives to warfarin. Despite the early risk of events in the periprocedural period, the overall safety event rates with the Watchman were similar to those with warfarin.
- © 2014 MD Conference Express®