Summary
Thromboembolism (TE) risk appears to be related to the burden of device-detected atrial tachyarrhythmias (AT) [Glotzer TV et al. Circ Arrhythm Electrophysiol 2009]. The investigators of the single-blinded Combined Use of BIOTRONIK Home Monitoring and Predefined Anticoagulation to Reduce Stroke Risk Trial [IMPACT; NCT00559988] hypothesized that a home monitoring—guided oral anticoagulation strategy, including initiation early after AT detection and withdrawal after a prespecified window without AT, might reduce TE and hemorrhage in patients with an implantable cardioverter-defibrillator or cardiac resynchronization therapy defibrillator device.
- Cardiology Clinical Trials
- Arrhythmias
- Thrombotic Disorders
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Arrhythmias
- Thrombotic Disorders
Thromboembolism (TE) risk appears to be related to the burden of device-detected atrial tachyarrhythmias (AT) [Glotzer TV et al. Circ Arrhythm Electrophysiol 2009]. The investigators of the single-blinded Combined Use of BIOTRONIK Home Monitoring and Predefined Anticoagulation to Reduce Stroke Risk Trial [IMPACT; NCT00559988], presented by Jonathan L. Halperin, MD, Mount Sinai Medical Center, New York, New York, USA, hypothesized that a home monitoring—guided oral anticoagulation (OAC) strategy, including initiation early after AT detection and withdrawal after a prespecified window without AT, might reduce TE and hemorrhage in patients with an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy defibrillator device.
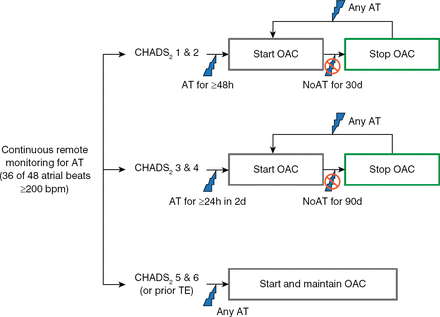
In total 2718 patients with CHADS2 risk scores ≥1 with ICD or CRT-D devices with home monitoring from 104 sites were randomized to home monitoring—guided OAC (n=1357) or physician-directed OAC without home monitoring (control; n=1361). The intervention group was assigned to remote monitoring for AT with a predefined plan for OAC based on the AT burden and CHADS2 score (Figure 1), whereas the control group was treated on the basis of in-office identification of AT and current standards of care for OAC.
Anticoagulation Protocol for the Intervention Group
AT=atrial tachyarrhythmia; OAC=oral anticoagulation.
The primary end point was the occurrence of the first stroke, systemic embolism, or major bleeding event. The secondary end points were all-cause mortality, all stroke, and AT burden.
The study was prematurely terminated on June 13, 2013, after the Data Monitoring Committee determined futility once 75% of expected events accrued. Cumulative follow-up included 5430 patient-years and median exposure of 701 days.
There were no differences in baseline characteristics except that 34.5% of the intervention group were taking antiplatelet therapy other than aspirin, compared with 30.7% of the control group (p=0.037). ATs were detected in 493 patients (36.3%) in the intervention group and 452 patients (33.2%) in the control group (p=0.0908). Of these, 126 patients in the intervention group and 115 in the control group had confirmed atrial fibrillation (AF) meeting the OAC criteria. The median CHADS2 score was 2 (p=0.544), and 64% of patients in each group had ICDs.
Among patients with AF meeting OAC criteria, 91 (72.2%) in the intervention group and 69 (60.0%) in the control group started OAC therapy. Forty-six patients (36.5%) in the intervention group and 29 (25.2%) in the control group stopped OACs. Mean days on OACs were 409 in the intervention group and 450 in the control group. Adherence to the OAC protocol was observed in 126 patients, 45.2% started OAC within the specified time frame, and the time within the therapeutic range was 61.2% in patients taking vitamin K antagonists.
There were no significant differences between the groups for any endpoint. The rate of the primary end point was 2.4% in the intervention group and 2.3% in the control group (HR, 1.06; p=0.732; Table 1). There was no temporal relationship between the occurrence of device-detected AT and TE events, suggesting that an accelerated OAC strategy did not improve the prevention of TE, said Dr. Halperin
Clinical Outcomes in the IMPACT Studya
Limitations of this study include suboptimal adherence with the OAC protocol and greater use of antiplatelet therapy in the intervention group. The low event rate limited the power to detect between-group differences in outcomes.
Starting and stopping OAC on the basis of device-detected AF did not improve clinical outcomes in the IMPACT study. Dr. Halperin concluded that the decision to start OAC therapy for device-detected AF should be based on a comprehensive clinical assessment of risk and benefit and that the absence of device-detected AF should not lead to the discontinuation of OAC.
- © 2014 MD Conference Express®