Summary
The shifting demographics, burden, and presentation of type 1 diabetes mellitus (T1DM) suggest that the associated costs and societal burden will increase during the next 20 to 30 years, according to data. Also discussed is the nomenclature of the classification and diagnosis of diabetes, as well as data indicating that genes and antibodies can be used for the enhanced prediction of T1DM.
- Diabetes Mellitus
- Prevention & Screening
- Diabetes Mellitus
- Prevention & Screening
- Endocrinology
- Diabetes & Metabolic Syndrome
The shifting demographics, burden, and presentation of type 1 diabetes mellitus (T1DM) suggest that the associated costs and societal burden will increase during the next 20 to 30 years, according to data presented by Dana Dabelea, MD, PhD, University of Colorado Denver, Denver, Colorado, USA.
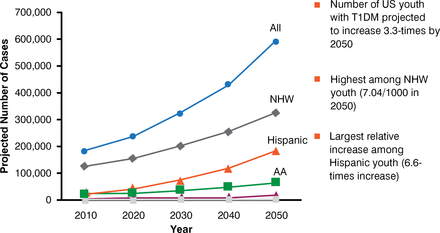
At the start of the 20th century, T1DM was rare and usually fatal. The typical patient was young, thin, and white. Since the mid-1900s, the incidence of T1DM has been increasing worldwide, with an incidence of 2% to 4% per year in 24 of 37 studies, and the average age at onset has been decreasing [Gale EA. Diabetes 2002]. Data from the SEARCH for Diabetes in Youth Project show that between 2001 and 2009, the prevalence of T1DM increased among all racial and ethnic groups except American Indians and among all ages except children < 5 years of age [Dabelea D, JAMA 2014]. In addition, the incidence of T1DM has increased significantly between 2002 and 2009 among non-Hispanic whites [Lawrence JM et al. Diabetes 2014]. The prevalence of T1DM is projected to increase substantially by 2050, with the largest relative increase being in Hispanic youth (Figure 1) [Imperatore G et al. Diabetes Care 2012]. Coupled with the increasing incidence of T1DM, there has been a decrease in the prevalence of high-risk human leukocyte antigen (HLA) genotypes and an increase in moderate- or low-risk HLA alleles, suggesting that environmental exposure is more able to trigger T1DM in children who were previously less genetically susceptible [Vehik K et al. Diabetes Care 2008].
Projected Number of Youth With Type 1 Diabetes Among U.S. Youth < 20 Years, by 2050
AA = African American; NHW = non-Hispanic white; T1DM = type 1 diabetes mellitus; US = United States.
Source: Imperatore G et al. Diabetes Care 2012.
Diagnosis of T1DM has become complicated. The SEARCH for Diabetes in Youth Project has recently recommended a new diabetes classification system based on 2 etiologic markers (autoimmunity and insulin sensitivity [IS]) that can distinguish 4 diabetic subtypes: autoimmune plus IS, autoimmune plus insulin resistant (IR), nonautoimmune plus IS, and nonautoimmune plus IR [Dabelea D et al. Diabetes Care 2011].
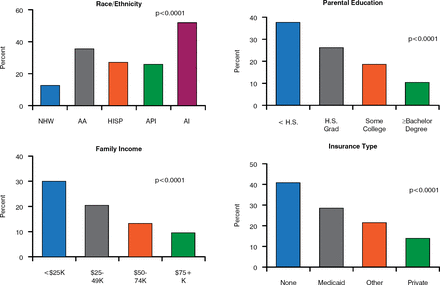
Risk factors for poor glycemic control, which is associated with poor prognosis, include being a minority, having parents with less education, lower family income, and less private health insurance (Figure 2) [Petitti DB et al. J Pediatr 2009]. Dr. Dabelea noted that there are substantial barriers to care among families with these socioeconomic characteristics that result in higher mortality and potentially avoidable hospitalization for complications.
Risk Factors for Poor Glycemic Control
AA = African American; AI = American Indian; API = Asian Pacific Islander; Hisp = Hispanic; H.S. = high school; Grad = graduated; NHW = non-Hispanic white.
Source: Petitti DB et al. J Pediatr 2009.
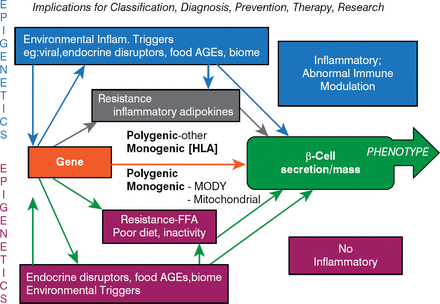
Stanley Schwartz, MD, Perlman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA, believes a new approach is needed to bring the nomenclature of the classification and diagnosis of diabetes in line with therapies. He recommends a β-cell-centric approach because it has implications for not only classification but also diagnosis, prevention, therapy, and research (Figure 3).
Pathogenic β-Cell-Centric Construct for Diabetes
AGE = advanced glycation end product; FFA = free fatty acids; HLA = human leukocyte antigen; Inflam = inflammation; MODY = maturity-onset diabetes of the young.
In the β-cell-centric construct, the phenotype is dependent on the genotype (the number of genes, the number of different genes, which genes, the nature of the genes, and the severity and intensity of expression). Dr. Schwartz believes that genotyping should be the standard diagnostic marker for a diagnosis of diabetes because this will ultimately lead to selecting the right drug for the right patient. He is currently pursuing a study to identify genes that are specific to latent autoimmune diabetes of adults.
Although glycemic criteria and C-peptides will remain of use with the β-cell-centric construct, the monogenetic causes of diabetes should be determined to develop new or use existing therapies to improve β-cell function and to pay more attention to inflammation as a contributor to the loss of functional β-cells. IR is clearly a target for therapy because it impairs β-cell function through lipo-and glucotoxicity, inflammation, and its adipocytokine effect. Potential therapies include weight reduction, anti-inflammatory drugs, quick-release bromocriptine, thiazolidinediones, and soon pro- and prebiotics.
There are environmental risk factors for diabetes that require a new focus within primary prevention [Atkinson MA, Eisenbarth GS. Lancet 2001]. These include genetic antibody screening, the potential use of immune modulators, environmental modulation, and the definition of risk factors. Diagnostic markers (genes, β-cell issues, inflammation, and IR) can be used to identify types of diabetes and potential therapies.
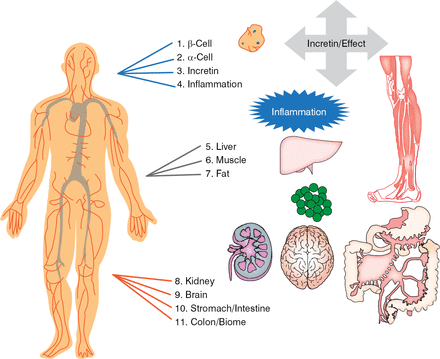
There are 11 targets for treatment of T1DM that decrease glucose and produce lipotoxicity (Figure 4). Four have a direct effect on β-cells (β-cell, α-cell glucagon defect, decreased incretin effect, and inflammation) that can be treated with incretin drugs, and 7 have an indirect effect on β-cells (liver, muscle, fat, kidney, brain, stomach and intestine, and colon-biome). The best approach is to treat as many of the 11 targets as needed to obtain the lowest possible levels of blood glucose and HbA1C without undue weight gain or hypoglycemia. Dr. Schwartz called for a new committee to reclassify diabetes based on the β-cell-centric construct for better prevention, diagnosis, and treatment of diabetes.
Eleven Targets for Diabetes Treatment
Anette-Gabriele Ziegler, MD, Institute of Diabetes Research, Technische Universität München, Munich, Germany, presented data indicating that genes and antibodies can be used for the enhanced prediction of the course of T1DM.
Checkpoints that define the natural history of T1DM can be used to determine the risk and rate of T1DM progression in children. The first checkpoint is the incidence of islet autoimmunity, which occurs between the ages of ∼ 9 months and 2 years (when it peaks). Early initiation of autoimmunity and rapid increases in autoantibody titers are associated with a progression to overt diabetes before puberty, emphasizing the importance of early life events in the development of T1DM [Ziegler AG et al. Diabetologia 2012].
The second checkpoint is the occurrence of multiple islet autoantibodies (ABs), which is highest 1 year after seroconversion. Progression to T1DM in children with multiple islet ABs was faster in those with islet AB seroconversion at < 3 years, the majority of whom progressed to diabetes during the next 15 years in a linear fashion [Ziegler AG et al. JAMA 2013]. Risk is persistently ∼ 11% per year.
The probability of developing islet autoimmunity is strongly linked to the HLA complex. HLA Class II genotypes and minor susceptibility genes can be used to predict T1DM and screen families. Other risk factors such as T-cells, type I interferon transcriptional signature [Ferreira RC et al. Diabetes 2014], and respiratory infection prior to seroconversion can indicate states of increased T1DM susceptibility.
Early peri- and postnatal environmental exposures such as maternal diabetes (decreases risk), early exposure to solid food (increases risk), and early infection load (increases risk) are associated with islet autoimmunity. Progression to multiple islet AB and T1DM are strongly associated with high-affinity insulin AB [Achenbach P et al. J Clin Invest 2004], pro-insulin reactive insulin autoantibodies (IAA), A8–13 reactive IAA, high-affinity glutamic acid decarboxylase autoantibodies (GADA) and middle- or c-terminal reactive GADA [Mayr A et al. Diabetes 2007].
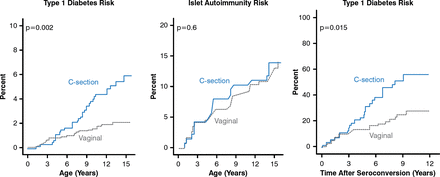
The rate of progression to symptomatic T1DM seems to be affected by age at initiation of islet autoimmunity, sex, and non-HLA susceptibility genes. Environmental exposures may also ‘pre-condition’ the rate of progression. Interestingly, children delivered by cesarean section have a > 2-times higher risk for T1DM than children born by vaginal delivery and have a faster progression to T1DM after seroconversion. Cesarean section does not increase the risk for islet AB (Figure 5) [Bonifacio E et al. Diabetes 2011].
Progression to Type 1 Diabetes in Children Born by Cesarean Versus Vaginal Delivery
Reproduced from Bonifacio et al. Cesarean Section and Interferon-Induced Helicase Gene Polymorphisms Combine to Increase Childhood Type 1 Diabetes Risk. Diabetes 20;60:3300–3306. With permission from the American Diabetes Association.
Population-based screening at birth for multiple islet AB and the combination of HLA and AB screening are useful approaches for detecting T1DM and preventing the progression to symptomatic T1DM.
- © 2014 MD Conference Express®