Summary
The EnligHTN renal denervation system reduced office blood pressure (BP) with no serious periprocedural adverse events over 24 months of follow-up. This article presents long-term data from the first-in-human Safety and Efficacy Study of Renal Artery Ablation in Resistant Hypertension Patients trial [EnligHTN I; NCT01438229].
- Cardiology Clinical Trials
- Hypertension & Kidney Disease
- Interventional Techniques & Devices
- Hypertensive Disease
- Renal Disease
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Hypertension & Kidney Disease
- Interventional Techniques & Devices
- Hypertensive Disease
- Renal Disease
The EnligHTN renal denervation system reduced office blood pressure (BP) with no serious periprocedural adverse events over 24 months of follow-up. Costas Tsioufis, MD, PhD, University of Athens, Athens, Greece, presented long-term data from the first-in-human Safety and Efficacy Study of Renal Artery Ablation in Resistant Hypertension Patients trial [EnligHTN I; NCT01438229].
The EnligHTN renal denervation system uses a multielectrode device to produce acute lesions with a predictable pattern. During the procedure, after being positioned proximal to the bifurcation of the renal artery, the basket is expanded, and a diagnostic check is performed to ensure electrode contact. Ablation is performed for 90 seconds per electrode. The basket is collapsed, pulled back 1 cm, then rotated and expanded, wherein the diagnostic check is performed, followed by ablation. The purpose of this study was to determine the long-term safety and efficacy of renal denervation with the EnligHTN renal denervation system.
In the open-label interventional EnligHTN I trial, 46 patients with resistant hypertension received renal denervation and were followed for 24 months. No control group was enrolled. Resistant hypertension was defined as office systolic BP of ≥160 mm Hg that did not respond to ≥3 concurrent antihypertensive medications at maximally tolerated doses for a minimum of 14 days before enrollment. Patients aged 18 to 80 years were excluded if they had a history of renal artery intervention, renal artery stenosis >30%, multiple main renal arteries, main renal arteries <4 mm in diameter or <20 mm in length, a glomerular filtration rate of <45 mL/minute/1.73m2, type 1 diabetes, identified cause of secondary hypertension, or significant valvular heart disease.
The primary objective was to evaluate all adverse events and office BP. At baseline, mean systolic and diastolic BP (SBP and DBP) were 176 and 96 mm Hg, respectively, with a mean number of antihypertensives of 4.7. In addition, 33% of patients had diabetes; 30% had sleep apnea; 59% had hyperlipidemia; 20% had coronary artery disease; and the mean body mass index was 32 kg/m2. The mean number of left and right renal artery ablations performed were 7.4 and 7.7, respectively, with a mean of 15 total ablations performed per patient. The mean procedure time was 34 minutes.
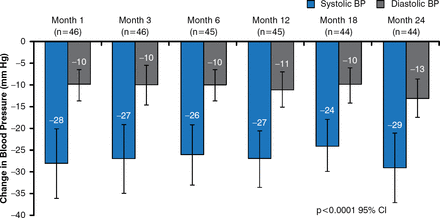
In the EnligHTN I trial, renal denervation resulted in a significant decrease in office SBP and DBP at 1 month that was maintained to 24 months (p<0.0001; Figure 1). Similarly, ambulatory BP was significantly decreased from baseline following renal artery denervation (p<0.0001). At 24 months, 77% of patients were considered to have responded to renal denervation, with a reduction of office SBP by >10 mm Hg from baseline. In addition, at 24 months, 39% of patients experienced an office SBP of <140 mm Hg.
Office Blood Pressure Following Renal Denervation by the EnligHTN Renal Denervation System
BP=blood pressure.
Reproduced with permission from C Tsioufis, MD.
Through 24 months of follow-up, there were no serious periprocedural events in the EnligHTN I trial. Serious device- or procedure-related events included 1 case of worsening preexisting proteinuria, 1 case of symptomatic hypotension, and 2 events in 1 patient of worsening of preexisting renal artery stenosis with a new stenotic lesion.
Prof. Tsioufis concluded that data from the EnligHTN I trial indicate that renal denervation with the EnligHTN system is effective in lowering office BP with an acceptable safety profile.
- © 2014 MD Conference Express®