Summary
Sjögren's syndrome (SS) is characterized by chronic inflammation occurring primarily in the exocrine glands. Better ways of defining and diagnosing patients with SS, monitoring patients and their disease activity, understanding the pathogenesis of the disease, and addressing the lack of effective evidence-based treatment are some of the challenges currently facing clinicians and patients. This is a very active field of research, with more than 800 articles published from 2013 to 2014. This article presents an overview of the latest SS research findings.
- Rheumatological Autoimmune Disorders
- Rheumatological Autoimmune Disorders
- Rheumatology
Sjogren's syndrome (SS) is characterized by chronic inflammation occurring primarily in the exocrine glands. First described in the 1930s by Henrik Sjogren, SS is most commonly characterized by dry eyes and mouth, severe fatigue, arthralgia, and myalgia. Better ways of defining and diagnosing patients with SS, monitoring patients and their disease activity, understanding the pathogenesis of the disease, and addressing the lack of effective evidence-based treatment are some of the challenges currently facing clinicians and patients. This is a very active field of research, with more than 800 articles published from 2013 to 2014. Marie Wahren-Herlenius, MD, PhD, Karolinska Institutet, Stockholm, Sweden, presented an overview of the latest SS research findings.
Women are much more likely than men to develop SS, with a peak incidence at 40 to 50 years of age. The clinical manifestations of SS are divided into glandular and extraglandular, and extraglandular features can affect nearly every organ in the body. The immune phenotype of SS includes increased memory B cells, hypergammaglobulinemia, anti-SSA/SSB antibodies, antinuclear antibodies, rheumatoid factor, the interferon (IFN) signature, lymphocytic infiltrates in salivary glands with germinal center formation, and increased levels of tumor necrosis factor, B-cell activating factor (BAFF), and IFN-a. The risk of lymphoma is 16 times higher in patients with SS, predominantly non-Hodgkin lymphoma B-cell type [Theander E et al. Ann Rheum Dis 2006].
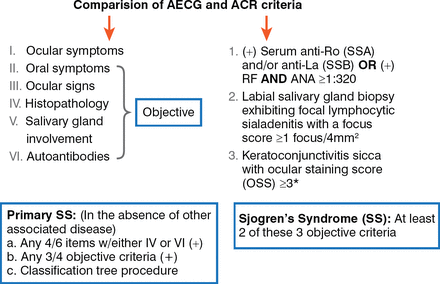
Classification criteria for SS continue to evolve. The American-European Consensus Group (AECG) revised its criteria in 2002 [Vitali C et al. Ann Rheum Dis 2002], and the American College of Rheumatology (ACR) criteria were published in 2012 [Shiboski SC et al. Arthr Care Res (Hoboken) 2012]. There are differences between the 2 groups (Figure 1). The ACR criteria are more complicated because they require evaluation in a specialized setting for the biopsy and ocular staining score. While most studies have demonstrated concordance between the 2 sets of criteria, there are cases that are overlooked by either set. Collaboration between the European League Against Rheumatism (EULAR) and the ACR is ongoing to develop universal validated consensus criteria.
Comparison of AECG and ACR Criteria
ACR=American College of Rheumatology; AECG=American-European Consensus Group; ANA=antinuclear antibodies; RF=rheumatoid factor;
SS=Sjogren's syndrome.
Lacrimal gland involvement in SS is evaluated by using tear flow via Schirmer's test and ocular surface stain and scoring. Salivary gland involvement is assessed by measuring unstimulated and stimulated salivary flow and salivary gland biopsy. The salivary gland biopsy is an invasive method that can lead to complications, so alternative methods are being studied. In parotid gland ultrasonography, ultrasound analysis of the parenchyma can detect the SS-associated appearance. Abnormal parotid ultrasonography has been shown to correlate with antibody-positive disease, hypergammaglobulinemia, clinical scores, and systemic disease. This method also appears to correlate with markers for lymphoma development [Theander E, Mandi T. Arthritis Res Care (Hoboken) 2014].
Two scales have been developed to assess disease activity in SS. The EULAR SS Patient Reported Index (ESSPRI) is rated by the patient and includes symptomatic features such as dryness, fatigue, and pain [Seror R et al. Ann Rheum Dis 2011]. Systemic features are rated by physicians in the EULAR SS Disease Activity Index (ESSDAI) [Seror R et al. Ann Rheum Dis 2010]. Validations studies of both assessments have been recently conducted. The ESSPRI was compared with the Sicca Symptoms Inventory and Profile of Fatigue and Discomfort, and the ESSDAI was compared with the SS Disease Activity Index and Sjogren's Systemic Clinical Activity Index. Analyses indicated that all scores had good to excellent reliability. The physician scores had greater sensitivity to change, with the ESSDAI having better construct and face validity and better discriminating power. The patients' scores had a smaller sensitivity to change, but the ESSPRI had better construct validity and sensitivity to change [Seror R et al. Ann Rheum Dis 2014]. “These scores have been very valuable to the field. We are now able to assess patient disease activity and compare between studies,” said Prof. Wahren-Herlenius.
Prof. Wahren-Herlenius also reviewed 4 recent clinical studies of the treatment of SS. In a randomized, double-blind, placebo-controlled trial, 26 patients with SS were treated for 4 weeks with placebo or the interleukin-1 receptor antagonist anakinra [Norheim KB et al. PLoS One 2012]. The primary outcome measure was a group-wise comparison of fatigue scores at Week 4 adjusted for baseline values. The study did not meet the primary outcome measure, as no significant differences were seen between the treatment groups. However, at 4 weeks, 6 of 12 (50%) anakinra-treated patients reported a 50% reduction in fatigue versus 1 of 13 (8%) patients taking placebo (p=0.03).
In another randomized, double-blind controlled trial, rituximab was tested against placebo in 120 patients with SS at 14 centers in France [Devauchelle-Pensec V et al. Ann Intern Med 2014]. Improvement at Week 24 of at least 30 mm in 2 of 4 visual analog scales (VAS) for fatigue, pain, dryness, and global disease was the primary outcome measure. While the study did not meet its primary end point, some significant alleviation of fatigue was seen at earlier time points.
In the prospective BELISS [Mariette X et al. Ann Rheum Dis 2013] Phase 2 open-label trial, 30 patients were treated with the anti-BAFF/BlyS drug belimumab. Patients with SS received belimumab 10 mg/kg at Weeks 0, 2, and 4 and then every 4 weeks to Week 24. The primary end point was measured at Week 28 and consisted of improvement in 2 of the following 4 items: 30% or more reduction in dryness, 30% or higher improvement in fatigue or pain VAS score, 30% or higher improvement in systemic activity VAS score, and/or greater than 25% improvement in B-cell activation biomarkers. Sixty percent of patients met the primary outcome measure.
The prospective open-label ASAP study [Meiners PM et al. Ann Rheum Dis 2014] was conducted in 15 patients who received intravenous abatacept infusions (10 mg/kg) on Days 1, 15, and 29 and every 4 weeks thereafter up to 24 weeks and were followed to Week 48. Disease activity was assessed with the ESSDAI and ESSPRI. The results were promising: Improvement in ESSDAI and ESSPRI scores was seen, with stable or slight increase in salivary gland function. In addition, most patients had a clinically relevant improvement in their well-being. While these trials were encouraging, concluded Prof. Wahren-Herlenius, the optimal drug remains to be found, which underscores the importance of defining pathogenic mechanisms.
- © 2014 MD Conference Express®