Summary
The term dystonia was first coined by Herman Oppenheim in 1911, who considered the core defect a problem with muscle tone. The definition has evolved throughout the years, with the latest being published in the 2013 Movement Disorder Society Consensus Update. They defined dystonia as “a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both” [Albanese A et al. Mov Disord 2013]. This article discusses this new classification, medical and nonsurgical treatments for dystonia, and current options for surgical treatment of dystonias.
- Interventional Techniques & Devices
- Extrapyramidal & Movement Disorders
- Interventional Techniques & Devices
- Extrapyramidal & Movement Disorders
- Neurology
According to Hyder A. Jinnah, MD, PhD, Emory University School of Medicine, Atlanta, Georgia, USA, the term dystonia was first coined by Herman Oppenheim in 1911, who considered the core defect a problem with muscle tone. The definition has evolved throughout the years, with the latest being published in the 2013 Movement Disorder Society Consensus Update. That international panel consisted of investigators with years of experience in this field who reviewed the definition and classification of dystonia. They defined dystonia as “a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both” [Albanese A et al. Mov Disord 2013].
The new classification describes dystonic movements as typically patterned, twisting, and sometimes tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation. The clinical manifestations are varied and include sustained, twisting, patterned postures; blepharospasm without sustained postures; dystonic tremor; laryngeal dystonia; and myoclonic dystonia. Overflow muscle activity may occur with task specificity, as in writer's cramp or musician's dystonia.
Whereas the original dystonia classification was divided into 3 categories—body distribution, age at onset, and etiology—the new system has 2 main categories, Axis I: Clinical Characteristics and Axis II: Etiology (Table 1).
Classification of Dystonia
The new classification has 2 separate axes because a classification scheme that is both clinically and biologically useful is needed. Axis I organizes knowledge of clinical features, aiding in diagnosis and treatment. Axis II organizes knowledge of biologic etiologies and identifies subgroups based on shared biologic mechanisms, enabling potential targeting of novel treatments to specific biologic mechanisms.
The new classification reflects important relationships between age at onset and body distribution. It includes 5 age groups, based on evidence from a series of recent studies showing a bimodal distribution with one peak at around 10 years and a second peak from age 40 to 60 years [De Carvalho Aguiar PM, Ozelius LJ. Lancet Neurol 2002]. Children tend to have generalized dystonia, whereas adults typically have focal dystonias. A recent study showed that most patients with generalized dystonia had their symptom onset before age 20 years, whereas in those with focal dystonia, it tended to develop at age ≥40 years, and segmental and multifocal dystonias typically occurred in between those ages [Xiromerisiou G et al. Mov Disord 2012].
Dr. Jinnah pointed out some common misconceptions about dystonia:
-
Co-contraction of antagonist muscles may occur but is not a defining feature.
-
Microstructural imaging defects occur in virtually all types.
-
Histopathologic abnormalities occur in certain subtypes of dystonia.
-
Dystonia is a genetic disorder—whereas many genes have been identified, >90% are sporadic or acquired.
MEDICAL AND NONSURGICAL THERAPIES
Alfredo Berardelli, MD, Neuromed Institute, Sapienza University of Rome, Rome, Italy, discussed medical and nonsurgical treatments for dystonia. Current treatments include symptomatic therapies that aim to reduce muscle overactivity and experimental treatments that are based on pathophysiologic abnormalities. A number of drugs have been used for medical treatment of dystonias (Table 2).
Medical Treatment for Generalized and Focal or Segmental Dystonias
Botulinum toxin revolutionized the treatment of focal and segmental dystonias, with oral drug treatment now being only occasionally used. There are 7 serotypes of botulinum toxin (BoNT), all of which inhibit acetylcholine release. BoNT type A is the most widely studied and used serotype. BoNT-A injected into the orbicularis oculi muscles is the treatment of choice for blepharospasm. Limitations include the need to repeat injections every 3 months and transient side effects. BoNT reduces the blinking rate in patients with increased blinking.
Some patients with oromandibular dystonia respond to BoNT, and 75% to 95% with laryngeal dystonia have improvement in voice symptoms in adductor spasmodic dystonia. The efficacy of BoNT for cervical dystonia has been established in several controlled trials. The main disadvantages include repeat injections and occasional side effects. Higher doses are needed throughout time to maintain efficacy. Patients may not respond to BoNT treatment due to inadequate dosage, inappropriate muscle selection, concomitant drug therapy, dynamic disease changes, or the development of neutralizing antibodies.
In addition to the above medications used to treat dystonia, other medical treatment options are available. Transcranial magnetic stimulation of the cortical motor areas and supraorbital nerve stimulation have also demonstrated some effectiveness in normalizing the pathophysiologic abnormalities of blepharospasm [Kranz G et al. Neurology 2010].
SURGICAL TREATMENT OPTIONS
Marwan Hariz, MD, University College London, London, United Kingdom, reviewed current options for surgical treatment of dystonias. According to Prof. Hariz, indications for surgical treatment include treatment-refractory, disabling mobile dystonia and refractory dystonic crisis, dystonic storm, and status dystonicus.
Modern surgical interventions include posteroventral pallidotomy and deep brain stimulation (DBS). Stimulation of the internal globus pallidus is associated with improvement of generalized dystonia and functional disability [Coubes P et al. Lancet 2000] and with improvement of cervical dystonia and dystonia-associated pain [Krauss JK et al. Lancet 1999]. Kupsch and colleagues [N Engl J Med 2006] reported that bilateral pallidal DBS for 3 months was more effective than sham stimulation in patients with primary generalized or segmental dystonia (p<0.001). In another study, DBS improved the dystonia movement score (p<0.001) and the disability score (p<0.001) at 12 months [Vidailhet M et al. N Engl J Med 2005]. The motor improvement with DBS observed at 1 year (51%) was maintained at 3 years (58%), as was improved quality of life [Vidailhet M et al. N Engl J Med 2007].
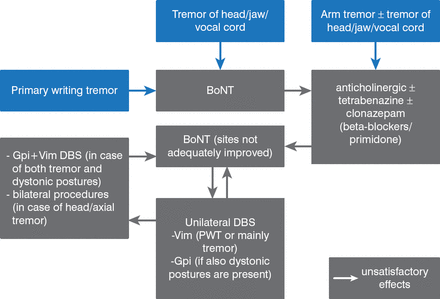
A meta-regression analysis of 157 studies found that patients with primary dystonias, myoclonus dystonia, heredodegenerative subtypes, and tardive dystonia have >50% improvement following DBS [Andrews C et al. J Neurol Neurosurg Psychiatry 2010]. Results of DBS for dystonic tremor are mixed. Based on data from 487 patients published in 43 papers, Fasano and colleagues [J Neurol Neurosurg Psychiatry 2013] created an algorithm for the management of dystonic tremor (Figure 1).
Algorithm for the Management of Dystonic Tremor
BoNT=botulinum toxin; DBS=deep brain stimulation.
Reproduced from Fasano A et al. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry 2014;85:759–769. With permission from BMJ Publishing Group Ltd.
Adverse effects of DBS include surgical or hardware complications such as intracerebral hemorrhage, seizures, and infections, as well as stimulation-induced effects, including flashes, muscle contractions, dysarthria, and mood changes. Patients can also develop akinesia of gait or Parkinsonian features.
Prof. Hariz stressed that patients with dystonic symptoms need to be identified and referred to movement disorder specialists. Patients with medically refractory, disabling, mobile, or tremulous dystonia should be referred to a functional neurosurgery team. He concluded that DBS is the primary surgical treatment for dystonias. DBS is better for primary than secondary dystonia and for mobile versus fixed dystonia, and it is more effective when performed early. It is important that patients have realistic expectations and receive postoperative support and care.
- © 2014 MD Conference Express®