Summary
The androgen receptor (AR) remains the most important target for metastatic castration-resistant prostate cancer (mCRPC). This article discusses new treatment modalities, including androgen synthesis inhibitors, chemotherapy, immunotherapy and radionuclide therapy.
- Reproductive Cancers
- Oncology
- Reproductive Cancers
The androgen receptor (AR) remains the most important target for metastatic castration-resistant prostate cancer (mCRPC). New treatment modalities, including androgen synthesis inhibitors, chemotherapy, immunotherapy and radionuclide therapy, were discussed in the Education Session “50 Years of Progress in Advanced Prostate Cancer” presented at the ASCO 2014 Annual Meeting.
NOVEL HORMONAL THERAPIES
Cora N. Sternberg, MD, Hospital San Camillo-Forlanini, Rome, Italy, session chair, presented “Hormonal Therapies in Prostate Cancer: The Old and the New,” and reviewed the history of hormone-based therapy for patients with prostate cancer. Despite advances in hormone treatments and a high response rate, most patients progress to CRPC, with a median survival of 4 years. CRPC is driven by alterations to the AR that are selected during therapy, including mutations, overexpression, gene amplification, and splice variants, as well as in response to signaling cross-talk and altered androgen levels produced by both the adrenal glands and the prostate tumor itself. Table 1 lists some of the novel anti-androgenic agents, their mechanisms of action, and their stage of development [Sternberg CN et al. 2014 ASCO Education Book].
Development of Selected Novel Agents That Block Testosterone Synthesis and Androgen Receptor Signaling
Dr. Sternberg said that the first Phase 3 trials of abiraterone and enzalutamide were conducted in patients with mCRPC who had received chemotherapy and other hormone therapies. The 4- to 5-month survival advantage for these oral hormone therapies was considered extraordinary at the time. These agents were then studied in patients who had not yet received chemotherapy. For example, the Phase 3 Safety and Efficacy Study of Oral MDV3100 in Chemotherapy-Naïve Patients With Progressive Metastatic Prostate Cancer [PREVAIL; Beer TM et al. New Engl J Med 2014] of enzalutamide versus placebo in mCRPC was stopped early by the independent data monitoring committee because of the efficacy of enzalutamide, which significantly reduced the risk of death, extended the time to chemotherapy, and significantly improved radiologic progression-free survival by 81%.
Cross-resistance among hormonal agents exists, and there is also cross-resistance with taxanes because they affect the AR. Therefore, it is necessary to ascertain how best to combine or sequence these drugs, and studies will address these questions. Patients with non-metastatic CRPC will be studied because it is not known how to treat these patients. There is also interest in developing nontoxic pre-chemotherapies and novel treatments that can be used earlier in the disease when a cure may be possible.
CHEMOTHERAPY IN RESISTANT PROSTATE CANCER
Daniel P. Petrylak, MD, Yale University Medical Center, New Haven, Connecticut, presented “Revising the Role of Chemotherapy for Resistant Prostate Cancer.” He reiterated that the best sequence of therapies for CRCP is unknown. The use of newer hormonal agents has allowed the initiation of chemotherapy to be delayed. Currently, docetaxel plus prednisone remains the standard of care as a first-line treatment for metastatic CRPC and should be considered for patients with high-risk, hormone-sensitive disease. None of the Phase 3 trials combining docetaxel with novel, targeted agents has shown better results than docetaxel plus prednisone.
Cabazitaxel has been shown to be more effective than mitoxantrone plus prednisone in patients with disease progression during and after treatment with a docetaxel-containing regimen [Bahl A et al. Ann Oncol 2013]. Cabazitaxel has been approved by the FDA as second-line chemotherapy [Jevtana Prescribing Information 2014], and further trials of this agent are ongoing.
IMMUNOTHERAPIES FOR PROSTATE CANCER
Ravi A. Madan, MD, National Institutes of Health, Bethesda, Maryland, discussed “Integrating Immunotherapies in Prostate Cancer Treatment.” Immunotherapeutic interventions in prostate cancer include therapeutic vaccines and immune checkpoint inhibitors. Therapeutic cancer vaccines generate a targeted anti-tumor immune response, are associated with minimal toxicities, may have effects that are delayed as compared with standard cytotoxic chemotherapy, and may be effective beyond the period of administration. The vaccine Sipuleucel-T is patient-specific and has been approved by the FDA for asymptomatic or minimally symptomatic mCRCP. The survival benefit is about 4 months but has no progression-free survival (PFS) advantage over the control treatment [Provenge Prescribing Information 2011]. Prostvac is an off-the-shelf vaccine containing tumor antigen and co-stimulatory molecule genes. In a Phase 2 trial, Prostvac increased overall survival (OS) by 8.5 months, although it also did not prolong PFS compared with control vector [Kantoff PW et al. J Clin Oncol 2010]. A Randomized, Double-blind, Phase 3 Efficacy Trial of PROSTVAC-V/F +/– GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer [Prospect; NCT01322490] is currently recruiting patients.
Checkpoint molecules regulate the immune system. Blocking these molecules can enhance T cell activity. Targets for immune checkpoint inhibitors include CTLA-4, which is expressed on T cells, PD1 (programmed death receptor 1), expressed on activated T and B cells, and PD ligand 1 (PDL-1), expressed on tumor cells. Immune checkpoint inhibitors have delayed and prolonged effects like vaccines; they also nonspecifically activate T cells and may cause autoimmune-related toxicities. Ipilimumab, a human monoclonal antibody against CTLA-4, did not show significantly increased survival (when combined with radiation for bone lesions) in a Phase 3 trial in patients with mCRPC that had progressed on docetaxel [Kwon ED et al. Lancet Oncol 2014]. A Phase 3 Study of Immunotherapy to Treat Advanced Prostate Cancer is ongoing [NCT01057810] and its objective is to evaluate whether chemotherapy-naïve patients with mCRPC have a longer OS with ipilimumab compared with placebo.
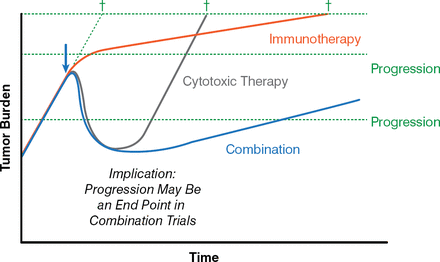
Combining immunotherapy with cytotoxic therapy was theorized as a potential way to improve time to progression. Cytotoxic chemotherapy rapidly kills tumor cells that later rebound as they become resistant, whereas immunotherapy may slow the growth rate rather than alter the short-term progression; immunotherapy may also have longer-term effects and may evolve as tumor antigen expression changes. It was suggested that integration of both approaches could yield the benefits of either treatment alone (Figure 1) [Madan RA et al. Semin Oncol 2012; Stein WD et al. Clin Cancer Res 2010].
Immunotherapy in Combination with Other Therapies May Improve Time to Progression
Adapted from Madan RA et al. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 2012;39(3):296–304. With permission from Elsevier.
RADIOISOTOPES FOR PROSTATE CANCER
Chris Parker, MD, The Institute of Cancer Research, London, United Kingdom, discussed “The Evolution of Radiopharmaceuticals in Prostate Cancer.” Strontium-89 is not widely used because of its hematologic toxicity, but it provides palliative benefits including reduced bone pain, fewer skeletal-related events (SREs), and improved quality of life (QoL) [James ND et al. J Clin Oncol 2013].
Radium-223 localizes to the bone and, as an alpha-emitter, was expected to cause less hematologic toxicity and be more lethal to cancer cells. In a Phase III Study of Radium-223 Dichloride in Patients With Symptomatic Hormone Refractory Prostate Cancer With Skeletal Metastases [ALSYMPCA; Parker C et al. New Engl J Med 2013], patients with symptomatic CRPC and 2 or more bone metastases were randomly assigned to radium-223 or placebo arms. Patients receiving radium-223 had better OS, delayed onset of SREs, better QoL, and fewer adverse events. Radium-223 has been approved by the United States Food and Drug Administration for the treatment of advanced prostate cancer, although Dr. Parker believes the current dose schedule could be improved and the agent tested in combination therapy.
Continued research is needed to understand the mechanisms of resistance and cross-resistance of currently approved therapies for advanced prostate cancer and to develop additional treatment modalities. Well-designed clinical trials will be required to determine the optimal sequencing and combinations for chemotherapeutic, immunologic, and radioisotopic agents in advanced prostate cancer.
The editors would like to thank the many members of the 2014 American Society of Clinical Oncology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®