Summary
A leadoff symposium at Heart Failure 2014 provided an update on the diagnosis of acute heart failure (AHF). This article provides an overview of the clinical assessment of AHF, which is an increasingly common cause of hospitalization and death, as well as point-of-care pulmonary ultrasound in assessing pulmonary congestion, biomarkers in the diagnosis of AHF, and an overview of the assessment and monitoring of congestion using noninvasive devices.
- Cardiology Genomics
- Imaging Modalities
- Heart Failure
- Cardiology Genomics
- Cardiology & Cardiovascular Medicine
- Imaging Modalities
- Heart Failure
A leadoff symposium at Heart Failure 2014 provided an update on the diagnosis of acute heart failure (AHF). Stavros Kakouros, MD, Fleming Hospital, Athens, Greece, provided an overview of the clinical assessment of AHF, which is an increasingly common cause of hospitalization and death. Early diagnosis is crucial in reducing both. Diagnosis can be challenging since AHF can be caused by any structural or functional cardiac disorder that impedes normal ventricle function. The majority of AHF cases are due to further deterioration in patients with chronic heart failure (HF) with preserved or reduced ejection fraction (pEF or rEF), although AHF can be the first manifestation of cardiac trouble.
Assessment for patients with apparent AHF should be immediate and should involve
-
▪ history—including age, sex, symptoms (dyspnea, orthopnea, fatigue), clinical signs (eg, edema), medical history (eg, coronary artery disease), medications (eg, diuretics), and comorbidities;
-
▪ clinical examination focusing on vital signs (blood pressure, heart rate, respiratory rate), appearance of jugular vein, heart and lungs, abdomen, and extremities;
-
▪ electrocardiography;
-
▪ radiograph of the chest;
-
▪ initial laboratory tests, including complete blood count, serum electrolytes, liver function, renal function, serum glucose, thyroid stimulating hormone, urinalysis, troponin, B-type natriuretic peptide (BNP), and arterial blood gases; and
-
▪ echocardiography.
Hospitalization based on AHF is typically due to
-
▪ not using medication,
-
▪ not adhering to a diet,
-
▪ acute myocardial ischemia,
-
▪ uncontrolled hypertension,
-
▪ atrial fibrillation or other arrhythmias,
-
▪ recent addition of negative inotropic compounds,
-
▪ pulmonary emboli,
-
▪ alcohol overconsumption or illicit drug use,
-
▪ thyroid dysfunction, or
-
▪ infections.
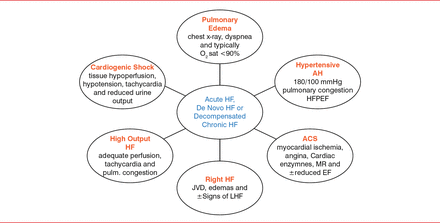
Six clinical phenotypes can be present (Figure 1).
Clinical Phenotypes of Acute Heart Failure at Presentation
ACS=acute coronary syndrome; AH=arterial hypertension; EF=ejection fraction; HF=heart failure; HF-pEF=heart failure with preserved ejection fraction; JVD=jugular vein distension; LHF=left heart failure; MR=mitral regurgitation.
Reproduced with permission from S Kaouros, MD.
The diagnosis of HF-rEF and HF-pEF requires symptoms and signs typical of HF. HF-rEF also requires reduced left ventricle ejection fraction, and HF-pEF also requires normal or mildly reduced left ventricle ejection fraction and nondilated left ventricle, as well as relevant structural heart disease (left ventricle hypertrophy, left atrial enlargement, or abnormal diastolic function). Clinical and hemodynamic congestion can be important in diagnosing AHF [Martin G et al. Chest 2002]. Blood pressure can be elevated, normal, or low. Hypotension is ominous, as it may reflect low cardiac output. Jugular vein distention can be key to diagnosis [Stevenson LW, Perloff JK. JAMA 1989]. BNP can also be crucial in diagnosing HF, but it should not be used alone.
Peter S. Pang, MD, MSc, Indiana University School of Medicine, Indianapolis, Indiana, USA, discussed point-of-care pulmonary ultrasound in assessing pulmonary congestion. Relief of congestion is a major goal of therapy [Mebazaa A et al. Crit Care Med 2010; Picano et al. Heart Fail Rev 2010], but a study of more than 150,000 patients indicated that about 20% can have incomplete relief from congestion at discharge [Yancy CW. Rev CV Med 2006].
Currently, there is no universally agreed-on assessment of the severity congestion that is easily reproducible and valid across users. Point-of-care ultrasound, which can be done with a handheld device, may be useful [Moore CL, Copel JA. N Engl J Med 2011]. B-lines are ultrasound lung comets that are reverberation artifacts from the pleural line. Nonetheless, they are valuable since they are caused by the presence of fluid in the lung, as in acute pulmonary edema. Absence of B-lines conclusively excludes cardiogenic pulmonary edema [Neskovic AN et al. Eur Heart J Cardiovasc Imaging 2013]. The number of B-lines determined from the ultrasound image relates to the severity of extravascular lung water [Picano E et al. J Am Soc Echocardiogr 2006]. Detecting and quantifying B-lines is a straightforward process that is easy to learn, with quality results being produced by personnel even after only 1 training session of <1 hour [Bedetti G et al. Cardiovasc Ultrasound 2006].
Christian Mueller, MD, University Hospital, Basel, Switzerland, discussed biomarkers in the diagnosis of AHF. Teasing out whether acute dyspnea is due to AHF or other conditions, such as chronic obstructive pulmonary disease, asthma, or pulmonary embolism, can be challenging given the lack of specificity and sensitivity or symptoms and signs. The consequence can be uncertainty in establishing whether a patient is at risk of HF, which increases mortality and costs of care [McCullough P et al. Circulation 2002].
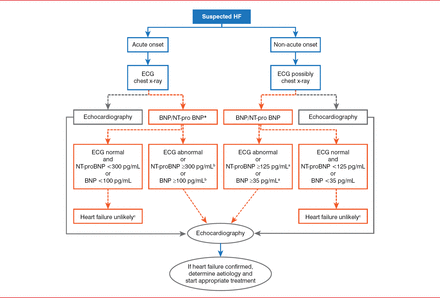
BNP is a marker of HF occurrence and severity, although it cannot discriminate among dysfunctions in left and right ventricles or heart valves [Maisel A et al. Eur J Heart Fail 2008]. It does improve diagnostic accuracy when used in the emergency department's detection of HF [Maisel A. N Engl J Med 2002]. BNP also improves patient management in terms of time to adequate therapy, and it reduces hospitalization and days of hospitalization, intensive care unit stay, and costs of care [Mueller C et al. N Engl J Med 2004]. BNP detection should not be used for diagnosis in patients suffering from shock, since their BNP levels can be elevated for reasons other than AHF. The latest European Society of Cardiology guidelines incorporate BNP detection into the diagnostic workup of patients with suspected AHF (Figure 2) [McMurray JJ et al. Eur Heart J 2012].
European Society of Cardiology Guidelines for Suspected Acute Heart Failure
*In the acute setting, MR-proANP may also be used (cut-off point 120 pmol/L, ie <120 pmol/L=HF unlikely); a Exclusion cut-off points for natriuretic peptides are chosen to minimize the false-negative rate while reducing unnecessary referrals for echocardiography; b Other causes of elevated natriuretic peptide levels in the acute setting are an acute coronary syndrome, atrial or ventricular arrhythmias, pulmonary embolism, and severe chronic obstructive pulmonary disease with elevated right heart pressures, renal failure, and sepsis. Other causes of an elevated natriuretic level in the non-acute setting are: old age (>75 years), atrial arrhythmias, left ventricular hypertrophy, chronic obstructive pulmonary disease, and chronic kidney disease; c Treatment may reduce natriuretic peptide concentration, and natriuretic peptide concentrations may not be markedly elevated in patients with HF-PEF.
BNP=B-type natriuretic peptide; ECG=electrocardiogram; ED=erectile dysfunction; HF=heart failure; MR-proANP=mid-regional pro atrial natriuretic peptide; NT-proBNP=N-terminal pro B-type natriuretic peptide.
Reproduced from McMurray JJ et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33(14):1787–1847. With permission from Oxford University Press.
Finally, it is clear that biomarkers can be valuable in obese patients, who are at increased risk of AHF, and that systemic infection can be a common trigger of HF.
Andrew Coates, MD, Monash University, Monash, Australia, provided a brief overview of the assessment and monitoring of congestion using noninvasive devices. Echo techniques, including echocardiography, are proven strategies. However, they can be lengthy and offer only indirect information. Use of natriuretic peptides is limited because of their half-lives, which may be insufficient for acute event monitoring, and because they are affected by patient characteristics such as age, sex, body weight, and renal function. Thoracic impedance monitoring has potential merit. Further out on the horizon are lung ultrasonography and remote dielectric sensing.
One of the reasons for the interest in noninvasive strategies is that invasive hemodynamic monitoring is not recommended by organizations, including the Heart Failure Society of America and the European Society of Cardiology. Furthermore, the reliability of individual clinical signs of congestion is not diagnostically robust [Gheorghiade M et al. Eur J Heart Fail 2010].
Noninvasive monitoring of HF-related congestion is still largely in the realm of clinical assessment. Routine use in diagnosis requires clinical trials. Novel technologies are potentially useful, if they are proved to add value to clinical assessments. For these technologies, time will tell.
- © 2014 MD Conference Express®