Summary
Several new agents are being explored for the management of heart failure (HF), which target different pathways involved in this complex disease. This article discusses human stresscopin, as well as a review of neurohumoral modulation, potential advantages of biased ligand at the angiotensin II type 1 receptor, soluble guanylate cyclase (sGC) stimulators, and potassium-binding polymers in HF.
- Heart Failure
- Cardiology & Cardiovascular Medicine
- Heart Failure
Several new agents are being explored for the management of heart failure (HF), which target different pathways involved in this complex disease.
Mihai Gheorghiade, MD, Northwestern University, Chicago, Illinois, USA, discussed human stresscopin, a corticotropin-releasing factor (CRF) type 2 receptor (CRFR2) selective agonist.
Effective therapies for patients with worsening HF represent an unmet need as patients with chronic HF are hospitalized often and have a high rate of post-discharge mortality. Worsening HF may require the use of inotropic drugs in patients with low cardiac output, but the currently available inotropes have deleterious effects on myocardial energetics and intracellular calcium, said Dr. Gheorghiade.
JNJ-39588146 is a synthetically manufactured acetate salt of human stresscopin, which binds selectively and with high affinity to CRFR2, a G-protein coupled receptor (GPCR). The effects of JNJ-39588146 on hemodynamics and neurohormone levels were studied in patients hospitalized with HF and having an ejection fraction (EF) less than or equal to 35% [Gheorghiade M et al. Eur J Heart Fail 2013]. Patients were included if they had a cardiac index greater than or equal to 2.5 L/min/m2 and pulmonary capillary wedge pressure (PCWP) greater than or equal to 20 mm Hg. In this double-blind, placebo-controlled trial, patients were randomly assigned in a 3:1 ratio to ascending IV doses of JNJ-39588146 (from 5 to 15 to 30 ng/kg/min) or to placebo administered in 60-minute intervals for 3 hours. JNJ-39588146 at 15 and 30 ng/kg/min increased the placebo-corrected cardiac index and reduced systemic vascular resistance without significant changes in heart rate (HR), systolic blood pressure, or pulmonary capillary wedge pressure (PCWP). This agent appears promising for stabilizing patients admitted with worsening chronic HF and low blood pressure due to low cardiac output.
John McMurray, University of Glasgow, Scotland, UK, continued with a case for neurohumoral modulation in the treatment of HF with a review of a first-in-class angiotensin receptor neprilysin inhibitor. HF can be considered a state of neurohumoral imbalance, he said, in which beneficial pathways that protect the heart from volume and pressure overload are diminished relative to harmful pathways (ie, renin-angiotensin-aldosteronesystem [RAAS]) that are being stimulated.
Augmenting the action of neurohumoral factors that are potentially beneficial in HF while inhibiting the action of detrimental mediators may represent a valuable therapeutic strategy, Prof. McMurray indicated. LCZ696, known as an angiotensin receptor neprilysin inhibitor (ARNi), is a molecular complex of an angiotensin receptor blocker (valsartan) and a neutral endopeptidase (NEP) inhibitor (AHU 377). The risk of angioedema, which prevented the neprilysin inhibitor omapatrilat from coming to market, is theoretically lower with LCZ696 than it is with the concomitant blockade of angiotensin-converting enzyme, aminopeptidase P, and neprilysin, which are involved in the breakdown of bradykinin, he explained.
LCZ696 has the potential to restore the appropriate balance of the RAAS and natriuretic peptides in HF. It reduces levels of N-terminal-pro-B-type natriuretic peptide and left atrial size, both of which have been associated with improved cardiovascular outcomes in HF. LCZ696 was the subject of a clinical trial called PARADIGM HF in which patients with chronic HF and reduced EF were randomly assigned to LCZ696 200 mg BID or enalapril 10 mg BID [McMurray JJV et al. Eur J Heart Fail 2013]. The Data Monitoring Committee unanimously recommended early closure of the study based on the benefit that was overwhelmingly statistically significant in favor of LCZ696 on the primary composite end point of cardiovascular death or HF hospitalization. The primary results of the PARADIGM HF trial are expected to be reported at the ESC Congress 2014.
Peter S. Pang, MD, Indiana University, Indianapolis, USA, explained the potential advantages of biased ligand at the angiotensin II type 1 receptor (AT1R) in acute HF, noting that AT1R activation in acute HF is both maladaptive and beneficial. The maladaptive effects are vasoconstriction and sodium and fluid retention, and the potential benefits are augmentation of cardiac contractility. Unbiased AT1R blockade would support tissue perfusion initially but could hasten cardiac deterioration, he said.
TRV027 is a β-arrestin-biased ligand that selectively activates a GPCR to augment cardiac contractility while promoting vasodilation and decreasing fluid retention. GPCRs are a large family of membrane receptors responsible for regulating various physiologic processes [Boerrigter et al. Circ Heart Fail 2012; Violin JD et al. J Pharmacol Exp Ther 2010]. TRV027 has a short half-life and is therefore its effects are rapidly reversible [Soergel DG et al. J Clin Pharmacol 2013]. It is cleared by proteolysis in the plasma, so dosage adjustments for renal insufficiency may not be necessary, said Dr. Pang. In a Phase 2a hemodynamic dose-escalation study (continuous infusion) in 33 subjects with stable HF and low EF, TRV027 reduced PCWP in patients with neurohormonal activation, and resulted in a sustained reduction in mean arterial pressure in patients with high plasma renin activity [Soergel DG et al. ACC 2013]. TRV027 is currently being studied in a Phase 2b study with patients with acute HF.
Burkert Pieske, Medical University of Graz, Graz, Austria, provided a rationale for the use of soluble guanylate cyclase (sGC) stimulators in HF. sGC is activated by endothelial nitric oxide synthase; however, this activation is impaired, and a deficiency of cyclic guanosine monophosphate (cGMP) results during states of oxidative stress. cGMP has a direct effect on myocardial and vascular function, and its deficiency induces dysfunction in both systems. In patients with HF with preserved EF, myocyte cGMP levels are reduced markedly, causing myocardial stiffness [van Heerebeek et al. Circulation 2012].
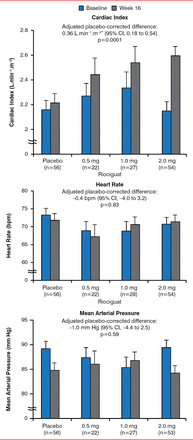
Augmenting cGMP signaling is recognized as a potential therapeutic strategy in patients with HF with preserved EF based on preclinical and clinical studies. In rats with hypertension induced by infusion of angiotensin II, sGC stimulation leads to a significant reduction in fibrosis and a reduction in the accumulation of collagen [Masuyama H et al. Hypertension 2006]. Stimulation of sGC using riociguat in patients with pulmonary hypertension secondary to systolic left ventricular dysfunction significantly increased cardiac index and other parameters of hemodynamic output at 16 weeks without a change in HR or mean arterial pressure (Figure 1) [Bonderman D et al. Circulation 2013]. Stimulation of sGC with vericiguat is currently being studied in HF in a program known as SOCRATES.
Change in Cardiac Index (A), Heart Rate (B) and MAP (C) at 16 Weeks
Reproduced from Bonderman D et al. Riociguat for Patients With Pulmonary Hypertension Caused by Systolic Left Ventricular Dysfunction: A Phase IIb Double-Blind, Randomized, Placebo-Controlled, Dose-Ranging Hemodynamic Study. Circulation 2013; 128:502–511. With permission from Lippincott Williams & Wilkins.
*On December 1, 2014, this was changed from L.min-1.m-2 to L.min−1.m−2.
Bertram Pitt, MD, University of Michigan, Ann Arbor, Michigan, USA, spoke of the use of potassium-binding polymers to facilitate the action of mineralocorticoid receptor antagonists (MRAs) in a broader population of patients with HF with reduced EF. Although MRAs have a proven survival benefit in patients with HF with reduced EF, many candidates for MRAs, especially those with concomitant chronic kidney disease (CKD), are not treated with these agents for fear of inducing hyperkalemia and worsening renal function, said Dr. Pitt.
Potassium-binding polymers bind potassium in the gastrointestinal tract, thereby reducing serum potassium levels. In a double-blind, placebo-controlled study known as PEARL-HF [Pitt B et al. Eur Heart J 2011], the polymeric potassium binder RLY5016 prevented hyperkalemia in patients with HF and a history of hyperkalemia or CKD receiving standard HF therapy and spironolactone, at a dosage of 25 to 50 mg/day.
- © 2014 MD Conference Express®