Summary
Rheumatoid arthritis patients treated with sarilumab (SAR) 150 or 200 mg Q2W or 100 or 150 mg QW were more than twice as likely to achieve an American College of Rheumatology 50 (ACR50) response after 12 weeks, compared with patients treated with placebo, and the benefit started appearing as early as 2 weeks. This article discusses a poster of a post hoc analysis from the Evaluation of SAR153191 (REGN88; Sarilumab) on Top of Methotrexate in Rheumatoid Arthritis Patients study [RA-MOBILITY; NCT01061736; Fleischmann R et al. EULAR 2013 (poster 0136)].
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatology
- Rheumatoid Arthritis
Rheumatoid arthritis (RA) patients treated with sarilumab (SAR) 150 or 200 mg Q2W or 100 or 150 mg QW were more than twice as likely to achieve an American College of Rheumatology 50 (ACR50) response after 12 weeks, compared with patients treated with placebo, and the benefit started appearing as early as 2 weeks. Roy Fleischmann, MD, Metroplex Clinical Research Center, Dallas, Texas, USA, presented a poster of a post hoc analysis from the Evaluation of SAR153191 (REGN88; Sarilumab) on Top of Methotrexate in Rheumatoid Arthritis Patients study [RA-MOBILITY; NCT01061736; Fleischmann R et al. EULAR 2013 (poster 0136)].
The need for new therapies is known, with only a minority of RA patients achieving a sustained clinical remission, despite the current treatment options [Kavanaugh A et al. Ann Rheum Dis 2013]. It has been shown that an elevated level of interleukin 6 (IL-6) drives inflammation in RA [Srirangan S, Choy EH. Ther Adv Musculoskelet Dis 2010], and work by a number of investigators has shown that blocking IL-6α with monoclonal antibodies (mABs) has been effective for some patients. A reduction in acute phase reactants has been shown with mABs in Phase I studies [Radin A et al. Arthritis Rheum 2010; Radin A et al. Ann Rheum Dis 2010].
This post hoc analysis was performed to evaluate the time-to-event of key outcomes by assessing differences in temporal patterns of the cumulative incidence of ACR20, ACR50, ACR70, and European League Against Rheumatology (EULAR) good plus moderate responses. A total of 306 adult patients with active RA for at least 3 months and with an inadequate response to methotrexate (MTX) in the Mobility Part A dose ranging study were included in this analysis. Baseline patient characteristics were similar among all groups (mean age at 52.2 years, 93.9% Caucasian, 79% women, mean disease duration of 7.8 years, and rheumatoid factor positivity 79.7%).
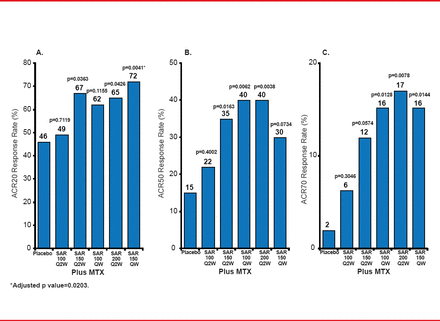
Researchers randomized the patients into 6 arms (n=51 or 52 each) to receive either SAR 100 mg Q2W plus MTX; SAR 150 mg Q2W plus MTX; SAR 100 mg QW plus MTX; SAR 200 mg Q2W plus MTX; SAR 150 mg QW plus MTX; or placebo plus MTX. All drug administration was subcutaneous. Figure 1 details the key results, including the primary endpoint of the ACR20 response rate at 12 weeks.
Primary and Secondary Outcomes (ACR Responses at Week 12)
ACR=American College of Rheumatology; Q2W=every other week; QW=weekly; SAR=sarilumab.
SAR doses of 150 mg Q2W or higher reduced RA signs and symptoms. Relative hazard ratios (HRs) favored 4 out of the 5 SAR doses compared with placebo, but not for 100 mg Q2W (Table 1). One death occurred from respiratory distress syndrome or a cerebrovascular accident in the SAR 100 mg Q2W group. Six of the 8 patients who experienced treatment-related adverse events in the two SAR groups discontinued treatment.
Likelihood of Achieving Efficacy Outcome With Sarilumab Compared With Placebo
The likelihood of achieving efficacy with an ACR50 response was high in 4 of 5 SAR doses but fell short for ACR20 responses.
- © 2013 MD Conference Express®