Summary
Due to multiple risks associated with magnetic resonance imaging (MRI) in patients with pacemakers, the American Heart Association published a consensus statement in 2007 indicating that MRI should only be used in patients with pacemakers if no other diagnostic techniques are available and if the benefits clearly outweigh the risks [Levine GN et al. Circulation 2007]. Risks include heating at the lead tips, image distortion, reed switch malfunction, asynchronous pacing, and death [Santini L et al. Pacing Clin Electrophysiol 2013]. This article discusses the safety of MRI in patients with pacemakers, the subcutaneous implantable cardiac defibrillator system, cardiac contractility modulators for patients with symptomatic heart failure, and the use of implanted devices for monitoring patient status, among other things.
- Heart Failure
- Imaging Modalities
- Interventional Techniques & Devices
- Cardiac Imaging Techniques
- Magnetic Resonance Imaging
- Cardiology & Cardiovascular Medicine
- Heart Failure
- Imaging Modalities
- Interventional Techniques & Devices
- Cardiac Imaging Techniques
- Magnetic Resonance Imaging
Due to multiple risks associated with magnetic resonance imaging (MRI) in patients with pacemakers, the American Heart Association published a consensus statement in 2007 indicating that MRI should only be used in patients with pacemakers if no other diagnostic techniques are available and if the benefits clearly outweigh the risks [Levine GN et al. Circulation 2007]. Risks include heating at the lead tips, image distortion, reed switch malfunction, asynchronous pacing, and death [Santini L et al. Pacing Clin Electrophysiol 2013]. Ali Oto, MD, Hacettepe University School of Medicine, Ankara, Turkey, discussed the safety of MRI in patients with pacemakers and the benefits of newer MRI conditional pacing systems.
Recently, two pacemakers have been approved by the US Food and Drug Administration (FDA) and an additional seven have been approved for use in Europe that are considered to be MRI conditional (Table 1). The status of MRI conditional indicates that there are no known safety issues. However, Prof. Oto highlighted that MRI conditional does not necessarily mean that the device is MRI safe [Levine GN et al. Circulation 2007].
Currently Available MRI-Conditional Pacemaker Devices
Several recent studies have demonstrated that MRI can be safely performed in patients with MRI conditional pacemakers. In a recent study of 464 patients with pacemakers, patients were randomized to undergo a 1.5T MRI or no MRI 9 to 12 weeks following device implantation [Wilkoff BL et al. Heart Rhythm 2011]. Participants that underwent the MRI did not experience complications in this study.
A small study compared patients with the Medtronic (n=112), St. Jude Medical (n=66), and Biotronik (n=72) pacemakers that underwent MRI with an average of 20 months of follow-up [Forleo GB et al. Int J Cardiol 2012]. The rate of complications was similar among the three groups, with lead impedance and capture threshold remaining stable during the follow-up period.
A trial evaluating the Advisa MRI pacing system randomized 263 patients 2:1 to receive MRI or not [Gimbel JR et al. Heart Rhythm 2013]. Both primary endpoints of MRI-related complications and pacing capture threshold changes prior to MRI to 1 month following MRI were met, suggesting the Advisa MRI pacing system is safe during MRI. In addition, 84% of left ventricular and 93% of right ventricular images were of excellent quality and only minor artifacts were associated with leads [Schwitter J et al. Heart Rhythm 2013]. Additional studies are ongoing.
Prof. Oto suggests that every patient should receive a MRI conditional pacing system if the costs and characteristics of the systems are equal [Santini L et al. Pacing Clin Electrophysiol 2013]. In addition, prior to MRI, the physician must ensure that the pacemaker is MRI conditional, an integrity check should be performed, and the pacemaker adjusted to MRI settings. Patients should be closely monitored during and shortly after the MRI. In addition, Prof. Oto noted that there are some ethical and legal considerations that should be taken into account when determining what pacing system to implant and if MRI approval should be given.
Gust H. Bardy, MD, Seattle Institute for Cardiac Research, Seattle, Washington, USA, discussed the subcutaneous implantable cardiac defibrillator (S-ICD) system. S-ICD is implanted based on anatomical landmarks, as the device is seated laterally over the fifth and sixth intercostal spaces and the lead follows the xiphoid muscle towards the midline and then passes to the left of the sternum. Three vectors are used for detection of rhythm, instead of beats. The algorithms work together and take into account heart rate, QRS width, and dynamic template matching with learning from previous beats.
Dr. Bardy states that the S-ICD system is simple to use with only four programmable parameters. The S-ICD system has an output of about 1400 volts, as compared with the 700 volts of a conventional transvenous system. However, Dr. Bardy points out that the actual impulse that reaches the heart is much lower with the S-ICD system than the transvenous system due to the skin acting as a block.
A recent study suggests that a subcutaneous defibrillator produces less heart tissue damage than a transvenous system [Jolley M et al. Heart Rhythm 2008]. The only absolute contraindication for the S-ICD system is patients with pre-existing unipolar pacemakers and relative contraindications include a monomorphic ventricular tachycardia (VT) <170 bpm, need for antibradycardia pacing, and evidential termination of VT by ATP.
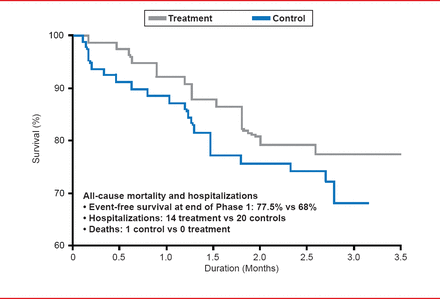
Carlo Pappone, MD, PhD, GVM Care and Research, Milan, Italy, discussed a cardiac contractility modulator (CCM) for patients with symptomatic heart failure (HF). Prof. Pappone highlighted several clinical trials that demonstrated safety and efficacy for the CCM system. In the FIX-CHF-4 trial [Borggrefe MM et al. Eur Heart J 2006], 164 HF patients with left ventricular dysfunction were randomized to receive CCM for 3 months followed by sham treatment for 3 months or sham treatment for 3 months followed by CCM for 3 months. Patients that received CCM experienced significantly improved exercise tolerance, quality of life, and fewer hospitalizations (Figure 1), as compared with the sham treatment.
Treatment With CCM Resulted in Fewer Hospitalizations.
Reproduced with permission from C Pappone, MD, PhD,
In the double-blinded FIX-HF-5 US feasibility study [Neelagaru SB et al. Heart Rhythm 2006], 49 patients received CCM and were randomized 1:1 for the device to be on for 6 months (n=25) or off for 6 months (n=24). In this study, 62% of participants in the treatment arm were hospitalized for any cause, as compared with 84% in the control arm. In addition, one death occurred in the control arm and none in the treatment arm. However, greater improved in ejection fraction, end-diastolic dimension, peak oxygen consumption (VO2), and anaerobic threshold was observed in the control group, as compared with the treatment arm.
In what Prof. Pappone considers to be the most important study, a controlled trial randomized 428 HF patients with a narrow QRS and an ejection fraction ≤35% to receive medical therapy and CCM or medical therapy alone [Kadish A et al. Am Heart J 2011]. Patients that received CCM experienced greater improvement in peak VO2 and quality of life, as compared with those patients that received only medical therapy. In addition, noninferiority of CCM was achieved, as 52% of patients that received CCM and 48% of patients that received medical therapy alone met the primary safety endpoint of all-cause mortality and hospitalizations (p=0.03).
Carsten Israel, MD, Evangelical Hospital Bielefeld, Bielefeld, Germany, discussed the use of implanted devices for monitoring patient status. Prof. Israel pointed out parameters in several systems that could provide useful information to the physician, that in some cases, would not have been noticed by the patient or physician otherwise. The OPTIVOL algorithm system detects impedance caused by lung edema, which can be an indicator of worsening heart failure. In some cases where the impedance signal may be unusual in appearance, it may indicate problems other than decompensated HF, such as anemia, lead dislodgement, lead malfunction, dialysis, or pericardial effusion. The Biotronik system also measures impedance, which can be averaged over 7 days. Due to the relationship of the leads and the right ventricular coil, impedance as measured by Biotronik may be correlated to left ventricular volume and stroke volume [Stahl C et al. Pacing Clin Electrophysiol 2009]. Modern devices offer further monitoring options such as heart rate variability or endocarial acceleration as markers for cardiac decompensation, or ST segment monitoring to detect (silent) ischemia.
Although in many cases additional studies are required, new technologies in implanted devices holds promise in improving patient outcomes, decreasing hospitalizations, and improving the ability of the physician to monitor patient status.
- © 2013 MD Conference Express®