Summary
Long-term data from the Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation trial [PROTECT AF; NCT00129545] indicate that left atrial appendage (LAA) closure with the Watchman provides superior protection from stroke compared with warfarin. The data also showed that Watchman LAA occlusion filter is associated with a 40% reduction in stroke/systemic embolism/cardiovascular (CV) death, a 60% reduction in CV mortality, and a 34% reduction in all-cause mortality.
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
Long-term data from the Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation trial [PROTECT AF; NCT00129545] indicate that left atrial appendage (LAA) closure with the Watchman provides superior protection from stroke compared with warfarin. The data, presented by Vivek Y. Reddy MD, Mount Sinai School of Medicine, New York, New York, USA, also showed that Watchman LAA occlusion filter is associated with a 40% reduction in stroke/systemic embolism (SE)/cardiovascular (CV) death, a 60% reduction in CV mortality, and a 34% reduction in all-cause mortality.
The PROTECT AF trial was a noninferiority/superiority randomized controlled trial conducted to determine whether the Watchman device could replace warfarin for stroke prevention in patients with nonvalvular AF and ≥1 CHADS2 risk factor. A total of 707 patients were randomly assigned in a 2:1 ratio to percutaneous closure of the LAA and subsequent discontinuation of warfarin (intervention; n=463) or to warfarin treatment with a target international normalized ratio (INR) between 2.0 and 3.0 (control; n=244) [Holmes DR et al. Lancet 2009]. The composite primary efficacy endpoint included stroke, SE, and CV death. Patients in the study were mostly white (∼91%) and male (70%), and the mean CHADS2 score was 2.2. About 20% of patients had experienced a stroke or transient ischemic attack prior to entering the study.
Two prior reports from PROTECT AF suggested that closure of the LAA is noninferior to warfarin and might provide an alternative strategy to chronic warfarin therapy for stroke prophylaxis in this group of patients [Holmes DR et al. Lancet 2009; Reddy VY et al. Circulation 2013]. However, both reports also indicated more primary safety events in the Watchman group (mainly periprocedural complications) than in the control group. Importantly, the PROTECT AF study also, for the first time, has implicated the LAA in the pathogenesis of stroke in AF [Reddy VY et al. Circulation 2013].
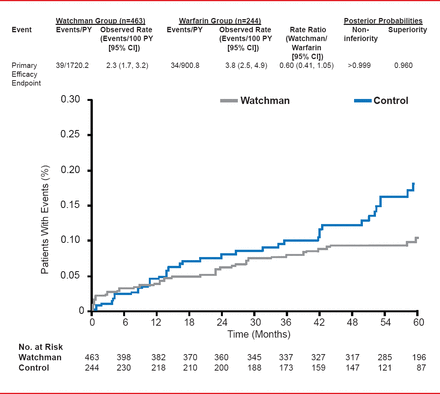
In the results presented by Dr. Reddy, the PROTECT AF participants were followed for a mean of 45 months (range, 0 to 77.5; aggregate 2621 patient-years). For the primary efficacy endpoint of stroke, SE, or CV or unexplained death, events per 100 patient-years (95% CI) were 2.3 (1.7 to 3.2) for the Watchman group versus 3.8 (2.5 to 4.9) for the warfarin group. The rate ratio (95% CI) was 0.60 (0.41 to 1.05) indicating not only noninferiority (posterior probability of >0.999) but, for the first time, superiority (posterior probability 96%).
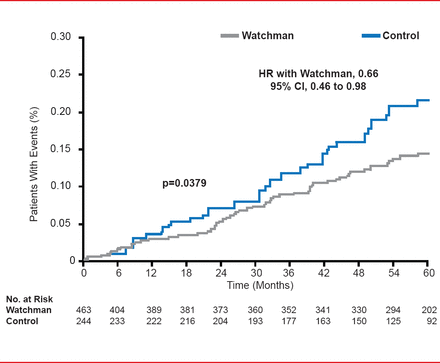
Relative risk according to subgroup analysis showed similar results, all favoring the Watchman group. All-cause mortality in the intent-to-treat population also favored the Watchman group (HR 0.66; 95% CI, 0.45 to 0.98; p=0.0379).
PROTECT AF: Primary Efficacy Endpoint
Reproduced with permission from VY Reddy, MD.
Intention-to-Treat: All-Cause Mortality
Reproduced with permission from VY Reddy, MD.
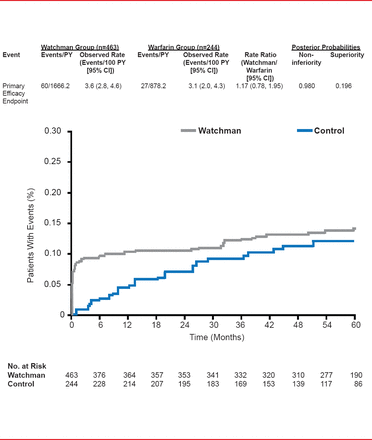
Unlike earlier results, in the 45-month analysis the results for the primary safety endpoint (a composite of serious pericardial effusion, major bleeding, procedure-related stroke, hemorrhagic stroke, and device embolization) were similar for the two approaches but with a bimodal distribution that diminished over time and with operator experience (RR, 1.17; 95% CI, 0.78 to 1.95).
Primary Safety Endpoint
Reproduced with permission from VY Reddy, MD.
“Over the course of more follow-up, we see that the amount of benefit hasn't really changed; what has changed is our certainty of this benefit really being true,” stated Dr. Reddy.
- © 2013 MD Conference Express®