Summary
A registry and a follow-up of patients using the LifeVest Wearable Cardiac Defibrillator (WCD), WEARIT-II, shows that the vest saves lives and can be safely used to bridge a decision for appropriate implantable cardioverter defibrillator therapy. The purpose of the study was to provide prospective data on the safety and efficacy of a bridging strategy with the WCD in a real-world setting. This article presents 18-month results for the first 882 patients enrolled in the United States from August 2011 through April 2013.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology
A registry and a follow-up of patients using the LifeVest Wearable Cardiac Defibrillator (WCD), WEARIT-II, shows that the vest saves lives and can be safely used to bridge a decision for appropriate implantable cardioverter defibrillator (ICD) therapy. Ilan Goldenberg, MD, University of Rochester, Rochester, New York, USA, presented 18-month results for the first 882 patients enrolled in the United States from August 2011 through April 2013.
The purpose of the study was to provide prospective data on the safety and efficacy of a bridging strategy with the WCD in a real-world setting. The WEARIT-II Registry design included acquisition of baseline data, wearing time of 2 to 6 months, acquisition of clinical and arrhythmic events during device usage, end of use reason evaluation, and a 12-month follow-up in patients with acquired, inherited, or congenital heart disease.
The patients and conditions under which the vest was used were diverse: in post-MI patients; following coronary revascularization; new onset dilated (nonischemic) cardiomyopathy; high-risk patients until stabilized; and inherited arrhythmic or congenital disorders. In contrast to an ICD, the availability of a response button on the LifeVest could be used to reduce inappropriate and unnecessary shocks that are not life-saving.
The need for improved selection of patients for primary ICD therapy was evident in both the MADIT-II [Moss AJ et al. N Engl J Med 2002] and MADIT-RIT trials [Moss AJ et al. N Engl J Med 2012]. The former found that only one third of patients received appropriate ICD therapy over 4 years of follow-up; the latter, that ICD programming to <200 bpm was associated with increased risk for inappropriate shock and mortality. In addition, the rate of appropriate ICD shocks was 4% (overall applied shock rate was three events per 100 patient-years).
Inappropriate shocks occurred in 0.3% of the Registry population, a rate significantly lower than those seen in the MADIT studies. Death occurred in 0.05% of all patients (three without WCD; one with WCD). The population-wide event rate was nine per 100 patient-years among those with WCD therapy for ventricular tachycardia/ventricular fibrillation. For sustained ventricular tachycardia, the event rate was 27 per 100 patient-years out of a total of 53 events. Notably, the latter arrhythmias were self-terminating, and therefore appropriately not treated by the LifeVest since the patients pressed a response button on the device to withhold therapy, a feature that is currently unavailable on the implantable defibrillator (which therefore delivers therapy according to prespecified programming even in a conscious patient).
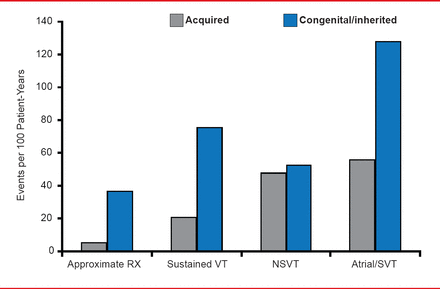
The rate of arrhythmic events was higher among patients with congenital or inherited heart disease than among those with acquired conditions (Figure 1).
Rate of Arrhythmic Events by Disease Etiology
VT=ventricular tachycardia; NSVT=nonsignificant ventricular tachycardia; SVT=supraventricular tachycardia.
Reproduced with permission from I Goldenberg, MD.
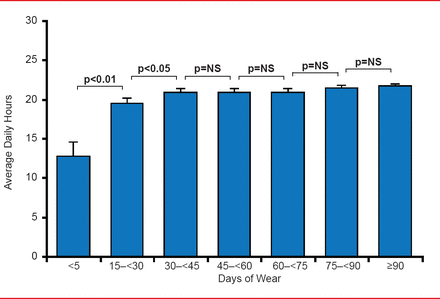
Patients wore the vests an average of 81±52 days. The mean compliance was 21±3 hours daily; the median daily compliance was 22 hours (interquartile range, 22 to 23 hours; Figure 2). Notably, compliance was significantly increased during the first month of wearing the LifeVest and remained high and stable thereafter.
WCD Compliance: Daily Hours
NS=nonsignificant; WCD=wearable cardiac defibrillator. Reproduced with permission from I Goldenberg, MD.
Importantly, following treatment with the LifeVest >40% of the patients did not require permanent implantation of an ICD due to improvement in ejection fraction. Therefore, real-world outcomes from the WEARIT-II registry show that the WCD is a safe way to give physicians time to make appropriate ICD therapy decisions. This bridge to therapeutic decision-making was shown to be safe and effective in several ways: (1) by safely terminating life-threatening arrhythmic events; (2) by avoiding unnecessary shock therapies for non-life-threatening ventricular tachyarrhythmias; and (3) by being associated with a very low rate of inappropriate therapies. As such, it opens the door to more targeted treatment for high-risk patients who suffer from congenital or acquired heart disease.
- © 2013 MD Conference Express®