Summary
Four conditions drive the clinical conundrum regarding the use of novel oral anticoagulants in patients also receiving dual antiplatelet therapy (DAPT): atrial fibrillation, left ventricular and intracardiac thrombus, mechanical valve prosthesis, and venous thrombotic disease and pulmonary embolism. Recurrent thrombotic events in patients on DAPT also could be considered a legitimate addition to this list. This article reviews the evidence for treatment approaches.
- Arrhythmias
- Thrombotic Disorders
- Cardiology & Cardiovascular Medicine
Four conditions drive the clinical conundrum regarding the use of novel oral anticoagulants (NOACs) in patients also receiving dual antiplatelet therapy (DAPT): atrial fibrillation (AF), left ventricular and intracardiac thrombus, mechanical valve prosthesis, and venous thrombotic disease and pulmonary embolism. Recurrent thrombotic events in patients on DAPT also could be considered a legitimate addition to this list. Paul W. Armstrong, MD, University of Alberta, Edmonton, Alberta, Canada, reviewed the evidence for treatment approaches.
Patients with acute coronary syndromes (ACS) and AF pose a particular risk and optimal therapy is unclear. About 2% to 21% of ACS patients have AF [Connolly S et al. Lancet 2006; Leon MB et al. N Engl J Med 1998]. Notably, compared with warfarin, aspirin and clopidogrel are more effective in ACS but less effective in AF.
The risk of events and bleeding is increased in patients with ACS and AF. A 5-fold increased risk of 30-day death in patients who had bleeding was found in some 34,146 patients in the OASIS registry, OASIS-2 trial, and CURE trial [Eikelboom JW et al. Circulation 2006]. A progressive and substantial risk of significant bleeding occurs as the number of antithrombotic (AT) and antiplatelet (AP) drugs increase; however, there was little effect on overall mortality in a Danish registry of nearly 40,812 patients, most of whom had acute myocardial infarction (MI) [Sorensen R et al. Lancet 2009]. By contrast, in another Danish registry of 11,480 patients with AF who had an MI or percutaneous coronary intervention (PCI), there was an inverse relationship between the number of thromboembolic events (cardiovascular death, MI, ischemic stroke) and the rate of fatal and nonfatal bleeding as the number of AT and AP drugs increased [Lamberts M et al. Circulation 2012]. The PRODIGY trial explored the optimal duration of DAPT and showed little additional benefit on clinical and ischemic endpoints with 24 months compared with 6 months of DAPT [Valgimigli M et al. Circulation 2012].
The WOEST study in ACS patients showed that the primary endpoint of any bleeding was lower with warfarin plus clopidogrel compared with these drugs plus aspirin (19.4% vs 44.4%, respectively; HR, 0.36; 95% CI, 0.26 to 0.50; p<0.001), and the incidence of the secondary composite clinical endpoint was lower with DAPT (11.1% vs 17.6% with triple therapy; HR, 0.60; 95% CI, 0.38 to 0.94; p=0.025) [De Wilde WJ et al. Lancet 2013]. Dr. Armstrong noted that although the study was underpowered for the secondary endpoint, the findings suggest it may be possible to forego aspirin in selected patients.
The likelihood of noncardiac surgery after stenting is a concern given the need for interruption of antithrombotic therapy. The rate of noncardiac surgery was 27.5% at 2 years in one cohort, with the incidence of major adverse cardiac events highest within the first 6 weeks (11.6%) and decreasing over time (3.5% at 12 to 24 months) [Hawn MT et al. JAMA 2013]. Recommendations based on these retrospective data are to 1) postpone nonurgent surgery until 6 months after stent implant; 2) use only one AP agent for urgent surgery <6 months; 3) consider short-term bridging with glycoprotein IIb/IIIa inhibitors or cangrelor if AP must be discontinued when stent thrombosis risk is highest; and 4) restart AP therapy soon after surgery once the bleeding risk returns toward the preoperative baseline [Hawn MT et al. JAMA 2013; Brilakis ES, Banerjee S. JAMA 2013].
Dr. Armstrong said that five questions must be addressed to determine the benefit-risk ratio for each patient:
-
What is the risk of the underlying condition?
-
What is the likely pattern and time frame of risk?
-
What is the risk of novel incremental treatment?
-
What is the likely pattern and time frame of risk reduction?
-
What is the time frame for intersection of perceived treatment risk and likely benefit?
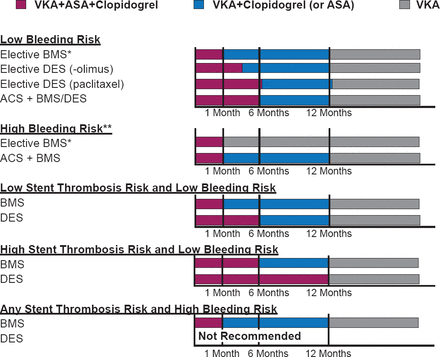
The recommendations for oral anticoagulants in ACS from the European Society of Cardiology (ESC) are shown in Figure 1 [Steg PG et al. Eur Heart J 2012], and for duration of triple therapy in AF after stenting are shown in Figure 2 [Verheugt F W et al. Circulation 2013].
ESC Recommendations for OACs in ACS
ASA=aspirin; AF=atrial fibrillation; DAPT=dual antiplatelet therapy; OAC=oral anticoagulant.
Reproduced from Steg PG et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;35(20):2569. With permission from Oxford University Press.
Recommendations for Duration of Triple Therapy After Stenting in AF
ACS=acute coronary syndromes; BMS=bare-metal stent; DES=drug-eluting stent; VKA=vitamin K antagonist.
Reproduced from Verheugt FW et al. Antithrombotic Therapy During and After Percutaneous Coronary Intervention in Patients With Atrial Fibrillation. Circulation 2013;128:2058–2061. With permission from Lippincott, Williams and Wilkins.
Dr. Armstrong sees on the horizon smarter dosing, in vitro physiologic monitoring, personalized medicine, effective antidotes, and a dynamic assessment of risk-benefit. The ongoing PIONEER AF-PCI study [NCT01830543] will help to answer the question of appropriate AT therapy in patients with ACS and AF.
- © 2013 MD Conference Express®