Summary
The promise of new-generation polymer, metallic, drug-eluting stents (DES) and biodegradable vascular scaffolds (BVS) are tantalizing, yet the question of whether they produce meaningful differences in vascular responses remains to be determined. This article discusses the development of DES with various polymers, as well as the early and late clinical advantages of bioabsorbable polymer DES.
- Interventional Techniques & Devices
- Interventional Techniques & Devices
- Cardiology
The promise of new-generation polymer, metallic, drug-eluting stents (DES) and biodegradable vascular scaffolds (BVS) are tantalizing, yet the question of whether they produce meaningful differences in vascular responses remains to be determined, according to Renu Virmani, MD, CVPath Institute, Inc., Gaithersburg, Maryland, USA.
Dr. Virmani focused on the development of DES with various polymers. In addition, she reviewed the vascular response to contemporary DES with durable polymers, polymer-free DES, and fully bioresorbable scaffolds.
A 2006 article on long-term effects of DES on coronary healing and mechanisms underlying late stent thrombosis described delayed arterial healing that occurred with DES as compared with bare-metal stents (BMS) [Joner M et al. J Am Coll Cardiol]. The authors reported that the cause of late-stent thrombosis (LST) in patients with DES was multifactorial but was related to delayed healing in addition to other clinical and procedural risk factors.
A paper published in 2008 reviewed progress with coronary BVS [Ramcharitar S, Serruys PW. Am J Cardiovasc Drugs]. The authors found that BVS had the advantage over DES of not leaving behind a permanent, metallic implant that could potentiate a thrombotic event and possibly preclude subsequent coronary surgery.
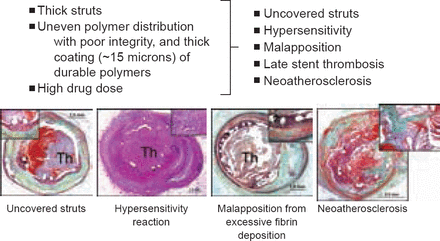
Three years later, a review by Nakazawa [J Cardiol 2011] reported that DES significantly reduced rates of restenosis compared with BMS. However, an increased risk of LST from device-related vascular abnormalities emerged as a major concern. Dr. Virmani explained that permanent polymer DES lead to endothelial dysfunction and predisposition to LST. Damaged enodothelium and LST seen with DES have been linked to thick stent struts, uneven polymer distribution with poor integrity and thick coating (∼15 microns) of durable polymers, high drug dose leading to uncovered struts, hypersensitivity reaction to the polymer, stent malapposition, and neoatherosclerosis (Figure 1).
Problems Encountered With Permanent Polymer Drug-Eluting Stents
Reproduced with permission from Renu Virami, MD.
However, advances in stent design and technology have been game-changers. A recent meta-analysis found that new biodegradable polymer DES are superior to first-generation DES when it comes to reducing target vessel revascularization, but are not better than newer-generation stents with a durable polymer [Bangalore S et al. BMJ 2013].
The authors noted that the latest trials of stents with bioabsorbable polymers have been noninferiority studies that have not been designed to show superiority over the new-generation durable-polymer DES. Currently, there does not appear to be any advantage with the bioabsorbable polymer stents compared to permanent polymer DES with regard to safety or efficacy.
The Randomized Clinical Comparison of Biomatrix Flex and Resolute Integrity trial [SORT-OUT VI; NCT01956448] also found that major cardiac event rates were extremely low—5.1% with the biolimus-eluting stent plus Nobori biodegradable polymer and 5.3% with the Resolute Integrity zotarolimus-eluting coronary stent.
As the treatment options increase and newer generation DES improve, the burden to demonstrate an advantage with BVS becomes more difficult. For the moment, this goal has remained somewhat elusive. In time, perhaps drug-eluting absorbable metal scaffolds will shift the equation and be shown to improve clinical outcomes.
Bangalore et al. [BMJ 2013] report that they have shown promising results in preclinical studies in a porcine coronary model, with almost full degradation at 2 to 3 years, and complete degradation at 4 years. When treating artherosclerotic lesions, the scaffolding provided by the stent is only required transiently. Over time, the vessel undergoes positive remodeling and develops morphology that is almost normal and has compact contractile smooth muscle cells concentrated toward the lumen.
Coronary intervention is a fast-changing field and our understanding of this area has significantly improved over the past 5 years. In the treatment of coronary disease, new materials and design technology offer the potential to bring new products to market and make current ones obsolete. Only time will tell which treatment options will pass muster in the future.
EARLY AND LATE ADVANTAGES: BIORESORBABLE POLYMER DRUG-ELUTING STENTS
Steady advances, based on outcomes from major randomized trials, have led the shift away from BMS to DES, and from DES to biodegradable polymer DES. Stephan Windecker, MD, Department of Cardiology, Bern University Hospital, Switzerland, discussed the early and late clinical advantages of bioabsorbable polymer DES.
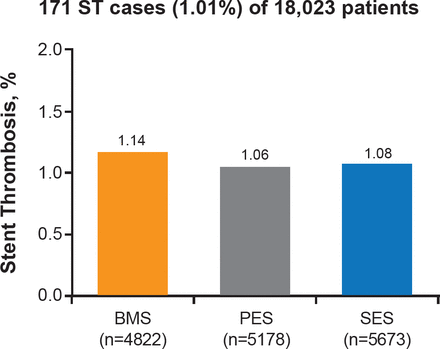
A meta-analysis compared early generation paclitaxel-eluting stents (PES) and sirolimus-eluting stents (SES) with BMS, and found no significant differences in mortality between the three options [Stettler C et al. Lancet 2007]. However, SES appeared to be clinically superior to BMS and PES at reducing risk of stent thrombosis at 1 year (Figure 2).
Stent Thrombosis at 1 Year With Early Generation Drug-Eluting Stents Versus Bare-Metal Stents
BMS=bare-metal stent; PES=paclitaxel-eluting stent; SES=sirolimus-eluting stent.
In a randomized clinical trial—Comparison of Biomatrix Versus Gazelle in ST-Elevation Myocardial Infarction (STEMI) [COMFORTABLE]—Räber et al. [JAMA 2012] compared the effect of biodegradable polymer biolimus-eluting stents versus BMS on cardiovascular events among patients with acute myocardial infarction (MI). Major adverse cardiac events at 1 year occurred in 24 patients (4.3%) who had biolimus-eluting stents with biodegradable polymer and 49 patients (8.7%) with BMS (HR, 0.49; 95% CI, 0.30 to 0.80; p=0.004).
A pooled analysis of individual patient data from three large randomized clinical trials—Rapamycin-Eluting Stents With Different Polymer Coating to Reduce Restenosis [ISAR-TEST-3]; the 3 Limus Agent Eluting Stents With Different Polymer Coating [ISAR-TEST-4]; and Limus Eluted From A Durable Versus Erodable Stent Coating [LEADERS]—compared biodegradable polymer DES with durable polymer SES, with clinical follow-up up to 4 years [Stefanini GG et al. Eur Heart J 2012].
Stefanini et al. found that at 4 years, the risk of target lesion revascularization was significantly lower among patients treated with biodegradable polymer DES versus durable polymer SES (HR, 0.82; 95% CI, 0.68 to 0.98; p=0.029). The risk of stent thrombosis was also significantly reduced with biodegradable polymer DES versus durable polymer SES (HR, 0.56; 95% CI, 0.35 to 0.90; p=0.015). These results were driven by a lower risk of very late stent thrombosis (HR, 0.22; 95% CI, 0.08 to 0.61; p=0.004). The authors noted that in a landmark analysis between 1 and 4 years, the incidence of MI was lower for patients treated with biodegradable polymer DES versus durable polymer SES (HR, 0.59; 95% CI, 0.73 to 0.95; p=0.031).
According to Prof. Windecker, newer-generation DES with thinner stent struts, novel durable or biodegradable polymer coatings, and new antiproliferative agents, have improved safety and efficacy outcomes compared with early-generation DES, making them the standard of care in all patient and lesion subsets.
However, as strut technology continues to develop, several novel DES with biodegradable polymer coating, polymer-free DES, and fully biodegradable coronary scaffolds have made their way to the clinical investigation stage. Stefanini et al. [Heart 2013] reported that preliminary angiographic and clinical evidence is promising, but it remains to be seen whether these novel devices are able to further improve the excellent safety and efficacy profile of currently used DES in patients with coronary artery disease undergoing PCI.
The search for greater safety and efficacy will continue, progressing in small steps and landmark studies. The field is rich in ongoing trials and first-in-man research; only time will tell whether this work pays off with incremental improvements or game-changing breakthroughs.
- © 2013 MD Conference Express®