Summary
Obesity causes changes in adipose tissue, the liver, and skeletal muscle that lead to systemic inflammation and insulin resistance. Obese individuals who have the ability to recruit new adipocytes develop adipocyte hyperplasia, whereas others have adipocyte hypertrophy due to impaired adipogenesis. The ability to recruit new adipocytes protects against ectopic fat accumulation in the abdomen, skeletal muscle, and liver.
- Obesity
- Cardiometabolic Disorder
- Insulin
- Inflammatory Disease
- Obesity
- Cardiometabolic Disorder
- Insulin
- Inflammatory Disease
Obesity causes changes in adipose tissue, the liver, and skeletal muscle that lead to systemic inflammation and insulin resistance. Obese individuals who have the ability to recruit new adipocytes develop adipocyte hyperplasia, whereas others have adipocyte hypertrophy due to impaired adipogenesis. According to Ulf Smith, MD, PhD, University of Gothenburg, Gothenburg, Sweden, the ability to recruit new adipocytes protects against ectopic fat accumulation in the abdomen, skeletal muscle, and liver.
Hypertrophic adipocytes secrete macrophage-attracting chemokines; free fatty acids (FFAs) released by insulin-resistant adipocytes activate the recruited macrophages. The macrophages can be classically or alternatively activated. In lean tissue, adipocytes secrete factors that promote alternative macrophage activation, leading to macrophage release of anti-inflammatory mediators and possible insulin sensitizing factors. Lipolysis is increased in obese tissue, resulting in secretion of FFAs and proinflammatory mediators that promote classical macrophage activation, leading to further inflammation and insulin resistance [Olefsky JM, Glass CK. Annu Rev Physiol 2010].
In a study of obese individuals with and without type 2 diabetes, analysis of baseline characteristics showed that both groups had the same total fat mass and abdominal subcutaneous fat, but participants with diabetes had significantly more abdominal visceral fat (2.9 kg vs 2.4 kg; p<0.001) and liver fat (8.3% vs 4.8%; p<0.001) than those without diabetes (Table 1) [Neeland IJ et al. JAMA 2012]. Among individuals without diabetes at baseline, those with the highest amount of visceral fat were at greatest risk for developing diabetes (p<0.001).
Baseline Characteristics of Obese Patients With and Without Incident Type 2 Diabetes
A study comparing lean and overweight individuals found that those with a genetic predisposition for type 2 diabetes had restricted adipogenesis and hypertrophic obesity even in the absence of obesity as defined by body mass index (BMI) [Arner P et al. PloS One 2011].
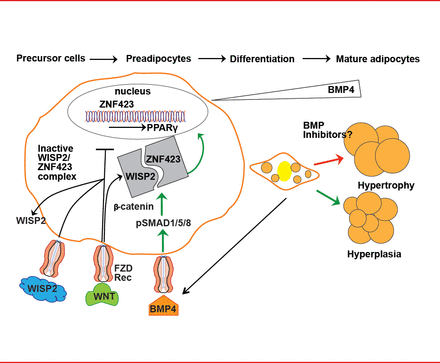
Restricted adipogenesis is not due to a lack of precursor cells but to an inability to recruit and differentiate subcutaneous preadipocytes [Isakson P et al. Diabetes 2009]. This inability is caused by inadequate signaling and activation of bone morphogenetic protein 4 (BMP4) and inadequate suppression of canonical Wnt signaling [Gustafson B et al. Diabetes 2013]. BMP4 is produced by preadipocytes and adipocytes, and it induces precursor cell commitment to the adipocyte lineage. Canonical Wnt prevents activation and differentiation of preadipocytes by peroxisome proliferator-activated receptor gamma (PPAR-γ). The Wnt-inducible secreted protein 2 (WISP2), expressed in preadipocytes, inhibits adipogenesis by forming a complex with the transcriptional activator of PPAR-γ, ZNF423. BMP4 dissociates this complex, resulting in entry of ZNF423 into the nucleus where it initiates PPAR-γ activation. WISP2 also directly inhibits PPAR-γ activation (Figure 1) [Gustafson B et al. Diabetes 2013].
Recruitment and Differentiation of Preadipocytes
Adapted from Gustafson B et al. Diabetes 2013.
These studies shed new light on the mechanisms of ectopic fat accumulation and development of hypertrophic obesity. Hypertrophic obesity is associated with dysregulated adipose tissue with reduced local and systemic insulin sensitivity regardless of the amount of body fat. The ability to recruit new subcutaneous adipocytes protects against ectopic fat accumulation, inflammation, and insulin resistance.
- © 2013 MD Conference Express®