Summary
Despite the use of statins in patients who are dyslipidemic, residual risk of cardiovascular disease remains increased in many individuals [Libby P. J Am Coll Cardiol 2005]. New therapies are therefore needed to enhance the current standard of care for patients with high cardiometabolic risk [Chapman MJ et al. Eur Heart J 2009]. Key opinion leaders discussed the role of other available options for elevating high-density lipoprotein cholesterol.
- Cardiometabolic Disorder
- Lipid Disorders
- Diabetes Mellitus
- Cardiology & Cardiovascular Medicine
- Cardiometabolic Disorder
- Lipid Disorders
- Diabetes Mellitus
Despite the use of statins in patients who are dyslipidemic, residual risk of cardiovascular disease (CVD) remains increased in many individuals [Libby P. J Am Coll Cardiol 2005]. New therapies are therefore needed to enhance the current standard of care for patients with high cardiometabolic risk [Chapman MJ et al. Eur Heart J 2009].
Key opinion leaders discussed the role of other available options for elevating high-density lipoprotein cholesterol (HDL-C). Jean-Pierre Després, PhD, Laval University, Quebec City, Quebec, Canada, explored the issue of raising HDL-C levels. Although low HDL-C levels predict coronary heart disease (CHD) risk in statin-treated patients [Kearney PM et al. Lancet 2008], he indicated that the solution to reducing CV risk is not as simple as increasing HDL-C levels, since both HDL-C particle size and concentration are independently associated with other CV risk factors, as well as risk for coronary artery disease [El Harchaoui K et al. Ann Intern Med 2009].
Lale Tokgözoǧlu, MD, Hacettepe University, Ankara, Turkey, presented data from trials on the therapeutic action of fibrates in atherogenic dyslipidemia. These agents are agonists of peroxisomal proliferator activated receptor-α, a transcription factor involved in fatty acid, lipid, and lipoprotein metabolism [Chapman MJ. Atherosclerosis 2003]. Prof. Tokgözoĝlu discussed data from two of the largest outcome studies in patients with type 2 diabetes mellitus (T2DM), the Fenofibrate Intervention and Event Lowering in Diabetes study [FIELD; Scott R et al. Diabetes Care 2009], and the Action to Control Cardiovascular Risk in Diabetes lipid trial [ACCORD; ACCORD Study Group. N Engl J Med 2010].
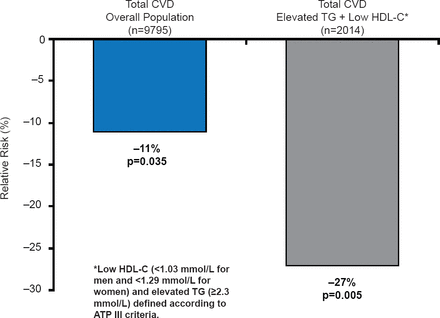
FIELD evaluated the efficacy of fenofibrate in subjects with T2DM [Scott R et al. Diabetes Care 2009]. Patients with severe dyslipidemia (triglycerides ≥2.3 mmol/L and low HDL-C were shown to be at the highest risk of CVD (17.8% over 5 years). Overall, fenofibrate did not significantly reduce the primary endpoint of CHD death or nonfatal myocardial infarction (MI; relative risk reduction [RRR],11%; p=0.16) [Sacks FM. Am J Cardiol 2008]. However, fenofibrate treatment reduced the incidence of CV events in patients with low HDL-C or hypertension, with the largest effect seen in those with marked dyslipidemia (RRR 27%; 95% CI, 9 to 42; p=0.005; Figure 1). Risk reductions were also greatest in patients without prior CVD [Scott R et al. Diabetes Care 2009].
Relative Risk of Cardiovascular Events in Patients in the FIELD Trial
CVD=cardiovascular disease; HDL-C=high-density lipoprotein cholesterol; TG=triglycerides.
The ACCORD Lipid Trial compared statin monotherapy with a statin plus fibrate on CV death, nonfatal MI and nonfatal stroke in >5500 subjects with T2DM [ACCORD Study Group. N Engl J Med 2010; MD Conference Express. ACC 2010]. Although there was no significant difference in the primary outcome (first occurrence of a major CV event, including nonfatal MI, nonfatal stroke, or death from CV causes) with fenofibrate compared with placebo (2.2% vs 2.4%; HR in the fenofibrate group, 0.92; 95% CI, 0.79 to 1.08; p=0.32), two hypothesis-generating observations in subgroup analyses warrant attention. Patients who had an elevated triglyceride level in the highest third of those studied (≥204 mg/dL [≥2.30 mmol/L]) and also had the lowest HDL-C levels (≤34 mg/dL [≤0.88 mmol/L]) had 31% fewer CV events with fenofibrate therapy (12.4% vs 17.3%; p=0.03) compared with all other patients (10.1% vs 10.1%; p=NS; p-interaction=0.057). Secondly, the effect of fenofibrate was significantly modified by sex (p-interaction=0.01), with a reduced CV event rate by 16% in men (11.2% vs 13.3%) compared with a 38% increase CV risk in women (9.1% vs 6.6%).
Although these data do not support routine addition of fenofibrate to background statin therapy to reduce CV risk in most patients with T2DM, they suggest potential benefit in helping to reduce residual risk in those with elevated triglycerides and low HDL-C, and perhaps in men but not women, that warrants further study [ACCORD Study Group. N Engl J Med 2010; Scott R et al. Diabetes Care 2009].
Philip Barter, MD, PhD, University of New South Wales, Sydney, Australia, reviewed data from the large, randomized Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events [HPS-2 THRIVE; Armitage J et al. Eur Heart J 2013] study that examined the use of combining extended-release niacin and laropiprant with statin treatment for the reduction of major CV events in more than 25,000 patients. The study demonstrated no significant reduction in maj or CV events with the addition of extended-release niacin/laropiprant to statin. Furthermore, serious adverse events (AEs), including increased risk of myopathy, gastrointestinal bleeding, stroke, and infection, were reported in ∼30 patients per 1000 throughout the almost 4-year course of the trial.
According to Raul D. Santos, MD, PhD, MSc, Heart Institute-InCor, University of São Paulo, São Paulo, Brazil, adjunctive therapy with ezetimibe has also been a controversial issue owing to randomized trials that have provided conflicting results about atherosclerotic plaque regression via carotid intima-media thickness, as well as a concern about a possible increase cancer risk [Peto R et al. N Engl J Med 2008].
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial [IMPROVE-IT; NCT00202878] is a randomized, clinical outcomes study comparing simvastatin 40 mg plus ezetimibe 10 mg with simvastatin 40 mg alone in over 18,000 patients with a recent acute coronary syndrome followed for a minimum of 2.5 years. The primary outcome is time to first major vascular event (CV death, nonfatal MI, hospital admission for unstable angina, revascularization >30 days, and nonfatal stroke). Data from the study are expected next year.
Low-dose supplementation with n-3 fatty acids, however, may lower the risk of major CV events in post-MI patients who are not treated with statins, said Daan Kromhout, MD, MPH, PhD, Wageningen University, Wageningen, The Netherlands. He presented data from the multicenter Alpha Omega Trial, the first, double-blind, placebo-controlled study to assess the efficacy of low doses of n-3 fatty acids (400 mg/day eicosapentaenoic acid [EPA]-docosahexaenoic acid [DHA], and/or 2 g/day α-linolenic acid [ALA]) on the risk of fatal and nonfatal major CV events [Eussen SRBM et al. Eur Heart J 2012].
In the trial overall, the primary endpoint of major CV events occurred in 14% of EPA-DHA versus 13% in placebo (adjusted HR, 1.05; 95% CI, 0.82 to 1.34; adjusted p=0.72). Although providing additional n-3 fatty acids to statin users did not reduce CV events, only 9% of statin nonusers who received EPA-DHA plus ALA experienced an event, compared with 18% in the placebo group (adjusted HR, 0.46; 95% CI, 0.21 to 1.01; p=0.051). For some post-MI patients who do not tolerate statins, omega-3 fatty acids may represent an alternative therapy to reduce major CV events that warrants further study [Eussen SRBM et al. Eur Heart J 2012].
Decisions about statin treatment for CVD prevention should be guided by anticipated benefits and potential AEs, stressed Guy De Backer, MD, PhD, University of Ghent, Ghent, Belgium. He presented data from a meta-analysis that evaluated results from 135 studies on different statins, to determine their comparative tolerability and harms [Naci H et al. Circ Cardiovasc Qual Outcomes 2013].
There was no difference in the occurrence of myalgia, creatine kinase elevation, cancer, and drug discontinuations due to AEs, between individual statins and control. Statin treatment in general, however, significantly increased the odds of developing diabetes (OR, 1.09; 95% CI, 1.02 to 1.16) and transaminase elevations (OR, 1.51; 95% CI, 1.24 to 1.84) [Naci H et al. Circ Cardiovasc Qual Outcomes 2013]. Given these data, clinicians should therefore advise patients of the modest risks prior to initiating statin therapy and monitor for development of these AEs. However, the implications of increased incident diabetes remain unclear in these studies, since statin treatment substantially reduced CV risk.
Alberico L. Catapano, MD, University of Milan, Italy, discussed data on proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, a new class of cholesterol-lowering drugs. PCSK9 is a protein in plasma that binds to low-density lipoprotein (LDL) receptors, resulting in their degradation such that fewer are present on the hepatic cell surface to remove excess LDL from the blood. PCSK9 inhibition therefore represents a new treatment strategy for dyslipidemia and associated CVD, and of the various classes of agents in development, two monoclonal antibodies, alirocumab and AMG 145, are in Phase 3, and several more are in Phase 2 development (Table 1).
PCSK9 Inhibitors in Development
Prof. Catapano presented data from the Phase 2 Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects study [GAUSS], a double-blind placebo-controlled trial in 160 patients intolerant of statins that evaluated the efficacy and safety of AMG 145 compared with ezetimibe control [Sullivan D et al. JAMA 2012; MD Conference Express. AHA 2012].
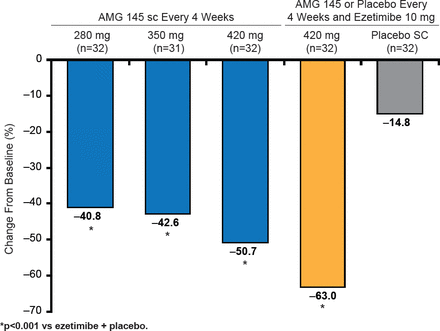
Patients were randomized to 1 of 5 groups (AMG 145 at doses of 280, 350, or 420 mg; AMG 145 at 420 mg plus ezetimibe 10 mg; ezetimibe 10 mg plus placebo) and were treated for 12 weeks. The primary endpoint was the percent change in LDL cholesterol (LDL-C) from baseline at 12 weeks [Sullivan D et al. JAMA 2012]. At Week 12, LDL-C levels in AMG 145-treated groups were significantly lower (p<0.001) than in ezetimibe-controlled group. Mean changes in LDL-C levels ranged from −40.8% to −50.7% in the AMG 145-only groups, compared with the AMG 145/ ezetimibe group at −63.0% and placebo at −14.8% (Figure 2) [Sullivan D et al. JAMA 2012].
AMG 145-Induced Changes in LDL-C Levels
Reproduced with permission from AL Catapano, MD.
Four serious AEs were reported with AMG 145 treatment versus zero with control. Myalgia was the most common treatment-associated AE (in 15.6% of subjects in the 280-mg group; 3.2% in the 350-mg group; 3.1% in the 420-mg group; 20.0% in the 420-mg AMG 145 plus ezetimibe group; and 3.1% in the ezetimibe plus placebo group). Further evaluation of the longer-term efficacy and safety of AMG 145 is underway in the FOURIER study [NCT01764633], a trial of 22,500 patients with prior CVD.
Børge G. Nordestgaard, MD, DMSc, Copenhagen University Hospital, Copenhagen, Denmark, discussed evidence suggesting that lipoprotein-a (Lp[a]) serves as an independent, genetic risk factor for CVD, with elevated levels associated with an increased risk of MI [Kamstrup PR et al. JAMA 2009].
In a study of patients without CVD, additional information on lipid-related markers (apolipoprotein B and A-I, Lp(a), or lipoprotein-associated phospholipase A2 mass) to total cholesterol and HDL-C improved CVD prediction [Di Angelantonio E et al. JAMA 2012]. Furthermore, extremely high Lp(a) levels can further improve CV risk prediction beyond conventional risk factors. In another study involving 8720 patients, Lp(a) levels ≥80th percentile (≥47 mg/dL) significantly improved MI (23%) and CHD (12%) risk prediction (p<0.001) [Kamstrup P et al. J Am Coll Cardiol 2013].
Prof. Nordestgaard concluded that while the relationship between Lp(a) and CV events requires further evaluation to determine its role as a marker of clinical risk, therapeutic options for its manipulation remain limited, and lipid-modifying strategies such as fibrates and statins have limited and variable effects [Nordestgaard BG et al. Eur Heart J 2010]. In the short-term (12 weeks), the PCSK9 inhibitor AMG 145 reduced Lp(a) by up to 32% compared with placebo [Desai NR et al. Circulation 2013]. Additional data with novel therapies to reduce Lp(a) over the long-term and to assess the impact on clinical outcomes are needed.
- © 2013 MD Conference Express®