Summary
Catheter-based renal artery denervation appears to result in sustained blood pressure (BP) reduction with a favorable safety profile in patients through 3 years with consistent benefit across age, diabetes status, and renal function. This article presents the final 3-year results from the Renal Denervation in Patients With Refractory Hypertension trial [Symplicity HTN-1; NCT00664638].
- Hypertensive Disease
- Renal Disease
- Hypertension & Kidney Disease
- Cardiology Clinical Trials
- Interventional Radiology
- Hypertensive Disease
- Cardiology & Cardiovascular Medicine
- Renal Disease
- Hypertension & Kidney Disease
- Cardiology Clinical Trials
- Interventional Radiology
Catheter-based renal artery denervation appears to result in sustained blood pressure (BP) reduction with a favorable safety profile in patients through 3 years with consistent benefit across age, diabetes status, and renal function, according to Henry Krum, MBBS, PhD, Monash University, Melbourne, Australia, who presented the final 3-year results from the Renal Denervation in Patients With Refractory Hypertension trial [Symplicity HTN-1; NCT00664638].
Although percutaneous renal denervation (RDN), an endovascular catheter-based procedure using radiofrequency energy, has been shown to successfully reduce BP for 1 year in patients with resistant hypertension [Krum H et al. Lancet 2009], its long-term efficacy may potentially be attenuated by sympathetic nerve regrowth and functional re-innervation.
The Symplicity HTN-1 was a series of pilot trials designed to evaluate the safety and BP-lowering efficacy of RDN using the Symplicity catheter system in refractory hypertension. These nonrandomized open-label studies were conducted among 19 centers in the United States, Australia, and Europe. Inclusion criteria were systolic BP (SBP) ≥160 mm Hg, despite full doses of ≥3 antihypertensive agents, and estimated glomerular filtration rate ≥45 mL/min. Exclusion criteria included type 1 diabetes, known secondary causes of hypertension, current clonidine, rilmenidine, or moxonidine therapy, and renovascular abnormalities.
The primary endpoints of the Simplicity HTN-1 were office BP and safety data before and at 1, 3, 6, 9, and 12 months after RDN. The secondary endpoints were the effects of RDN on renal noradrenaline spillover and renal function.
Of the 153 individuals enrolled, 65 patients (42.5%) were not included in the final analytic cohort because of missing baseline BP data, withdrawal of consent, loss to follow-up, or death. Results were presented for the remaining 88 patients (58%) who successfully completed the 36-month study.
In the 58% of patients who completed the study through to 36 months, renal function was demonstrated to remain stable, and few significant late-stage adverse events were reported (Table 1).
Late-Stage Adverse Events
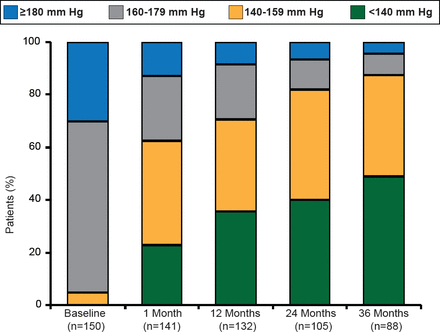
RDN was associated with significant (p<0.01) and sustained BP reductions (mean —32/—14 mm Hg) in patients who completed the study to 36-month follow-up. Still further, 50% of patients were able to achieve a target SBP <140 mm Hg (Figure 1). The BP reduction associated with RDN was consistent regardless of patient age, diabetes status, and baseline renal function.
Changes in SBP Through 36 Months
Reproduced with permission from H Krum, MBBS, PhD.
Prof. Krum concluded that Symplicity HTN-1 is the first and longest running clinical trial for RDN to date, comprising the largest cohort of patients. Although the proportion of the cohort with follow up (n=88 of 153) was limited and longer-term evaluation of this therapy in blinded control trials is required, the results of this study suggest that RDN has a favorable safety profile and sustained BP-lowering over 36 months in patients with refractory hypertension.
- © 2013 MD Conference Express®