Summary
This article presents pooled data from three clinical trials of cangrelor, an intravenous adenosine diphosphate-receptor antagonist in patients undergoing percutaneous coronary intervention (PCI) demonstrating significant reductions in thrombotic complications without an increase in major bleeding. The efficacy of the novel, investigational agent cangrelor has been evaluated in three randomized, double-blind, clinical trials against clopidogrel or placebo in patients during and after PCI: CHAMPION PHOENIX [Bhatt DL et al. N Engl J Med 2013]; CHAMPION PLATFORM [Bhatt DL et al. N Engl J Med 2009]; and CHAMPION PCI [Harrington RA et al. N Engl J Med 2009].
- Thrombotic Disorders
- Myocardial Infarction
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Thrombotic Disorders
- Myocardial Infarction
- Interventional Techniques & Devices
- Cardiology Clinical Trials
Christian W. Hamm, MD, PhD, Kerckhoff Heart and Thorax Center, Bad Nauheim, Germany, presented pooled data from three clinical trials of cangrelor, an intravenous adenosine diphosphate (ADP)-receptor antagonist in patients undergoing percutaneous coronary intervention (PCI) demonstrating significant reductions in thrombotic complications without an increase in major bleeding.
The efficacy of the novel, investigational agent cangrelor, a potent intravenous ADP-receptor antagonist with fast onset and short half-life of 3 to 6 minutes, has been evaluated in three randomized, double-blind, clinical trials against clopidogrel or placebo in patients during and after PCI: CHAMPION PHOENIX [Bhatt DL et al. N Engl J Med 2013]; CHAMPION PLATFORM [Bhatt DL et al. N Engl J Med 2009]; and CHAMPION PCI [Harrington RA et al. N Engl J Med 2009].
Prof. Hamm discussed the results of a meta-analysis of 24,910 patients enrolled in the CHAMPION program. The primary endpoint of the study was the composite of death from any cause, myocardial infarction (MI), ischemia-driven revascularization (IDR), or stent thrombosis (ST) at 48 hours. Secondary endpoints included ST at 48 hours and the composite endpoint of death/MI/IDR at 48 hours. The primary safety endpoint was GUSTO severe bleeding at 48 hours [Steg PG et al. Lancet 2013].
The efficacy analysis included patients (72% male; mean age 63 years) undergoing PCI for ST-elevation myocardial infarction (STEMI; 11.6%), non-ST elevation acute coronary syndromes (ACS; 57.4%), and stable coronary artery disease (31.0%) [Steg PG et al. Lancet 2013].
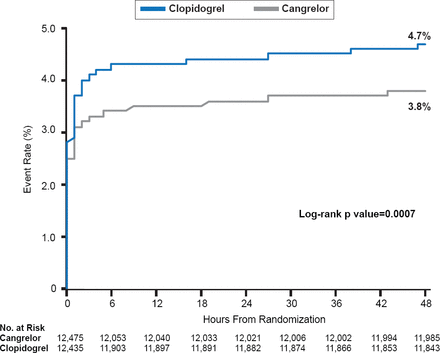
Among patients undergoing PCI, cangrelor was associated with a significant 19% relative reduction in the death/MI/IDR/ST at 48 hours compared with control (clopidogrel or placebo; 3.8% vs 4.7%; OR, 0.81; 95% CI, 0.71 to 0.91; p=0.0007; Figure 1) [Steg PG et al. Lancet 2013].
Rate of Primary Efficacy Endpoint in Cangrelor Versus Clopidogrel Groups
Reproduced from Steg PG et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013. With permission from Elsevier.
The rate of ST at 48 hours was reduced by 41% with cangrelor compared with control (0.5% vs 0.8%; OR, 0.59; 95% CI, 0.43 to 0.80; p=0.0008). There was no significant difference in GUSTO severe bleeding, the primary safety endpoint, GUSTO moderate bleeding, or in the rate of blood transfusions between cangrelor and control groups. The rate of GUSTO mild bleeding, however, was increased with cangrelor treatment (16.8% vs 13.0%; p<0.0001) [Steg PG et al. Lancet 2013].
Prof. Hamm noted that follow-up was limited to 30 days because this corresponded to data that were available from the CHAMPION PHOENIX study [Bhatt DL et al. N Engl J Med 2013]. Despite this minor limitation in the dataset, he concluded that the results of this analysis suggest intravenous cangrelor may represent a viable treatment option to reduce periprocedural thrombotic complications across the range of PCIs, including patients with STEMI, non-ST elevation ACS and stable angina [Steg PG et al. Lancet 2013].
- © 2013 MD Conference Express®