Summary
The primary goal of hypertension management is to reduce associated morbidity and mortality. This article discussed the differences in mortality and morbidity outcomes for angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Specific trials include ACCOMPLISH, ASCOT-BPLA, EUROPA, VALUE, VALIANT, ONTARGET, and ELITE II.
- Hypertensive Disease
- Hypertensive Disease
- Cardiology
The primary goal of hypertension management is to reduce associated morbidity and mortality. Victor Elliott, MBBS, DM, University Hospital of the West Indies, Kingston, Jamaica, West Indies, discussed the differences in mortality and morbidity outcomes for angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs).
A recent meta-analysis of 20 randomized controlled clinical trials in which subjects were randomly assigned to treatment with an ACE inhibitor, an ARB, or control demonstrated a significantly better treatment effect in favor of ACE inhibition (p value for heterogeneity 0.036). ACE inhibitors were associated with a 10% reduction in all-cause mortality (HR, 0.90; 95% CI, 0.84 to 0.97; p=0.004). There was no mortality reduction with ARB treatment (HR, 0.99; 95% CI, 0.94 to 1.04; p=0.683) [van Vark LC et al. Eur Heart J 2012].
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension trial [ACCOMPLISH] evaluated whether treatment with the combination of an ACE inhibitor and a dihydropyridine calcium-channel blocker (CCB) would be more effective in reducing the rate of cardiovacular (CV) events (defined as the composite of death from CV causes, nonfatal myocardial infarction [MI], nonfatal stroke, hospitalization for angina, resuscitation after sudden cardiac arrest, or coronary revascularization) than treatment with an ACE inhibitor and a thiazide diuretic. After 36 weeks, the benazepril-amlodipine combination was superior to benazepril and hydrochlorothiazide in reducing the composite endpoint (9.6% vs 11.8%; HR, 0.80; 95% CI, 0.72 to 0.90; p<0.001) among patients with hypertension who were at high risk for such events [Jamerson K et al. N Engl J Med 2008].
The Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm [ASCOT-BPLA] randomized patients to either amlodipine 5 to 10 mg adding perindopril 4 to 8 mg as needed (n=9639) or atenolol 50 to 100 mg plus bendroflumethiazide 1.25 to 2.5 mg and potassium as needed (n=9618). The primary endpoint was the composite of coronary heart deaths or nonfatal MI (including silent MI). Although not statistically significant, there was a weak trend toward fewer events in the patients treated with CCB and ACE inibitor (429 vs 474 events; unadjusted HR, 0.90; 95% CI, 0.79 to 1.02; p=0.1052). Fewer subjects treated with a combination of perindropil and amlodipine had fatal and nonfatal strokes (327 vs 422 events; HR, 0.77; 95% CI, 0.66 to 0.89; p=0.0003), total CV events and procedures (1362 vs 1602 events; HR, 0.84; 095% CI, 78 to 0.90; p<0.0001), and all-cause mortality (738 vs 820 events; HR, 0.89; 95% CI, 0.81 to 0.99; p=0.025) compared with subjects treated with the β-blocker atenolol and bendroflumethiazide [Dahlöf B et al. Lancet 2005].
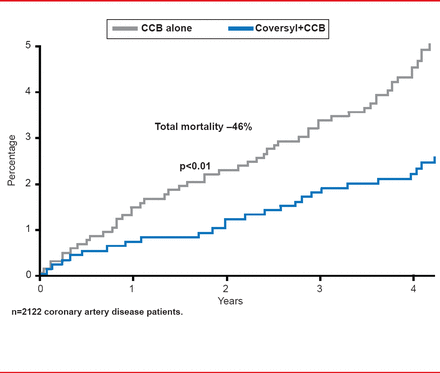
Perindopril-based strategies are also effective in patients with coronary artery disease. Results of a post hoc analysis from the EUROPA study showed that adding perindopril to a CCB has significant supplementary impact on CV mortality, nonfatal MI, and resuscitated cardiac arrest (HR, 0.65; 95% CI, 0.45 to 0.92; p<0.05) and mortality (HR, 0.54; 95% CI, 0.34 to 0.86; p<0.01 versus placebo; Figure 1) [Bertrand ME et al. Am Heart J 2010].
The objective of the Valsartan Antihypertensive Long-Term Use Evaluation trial [VALUE] was to assess whether the ARB valsartan would reduce cardiac morbidity and mortality more than amlodipine while providing the same level of blood pressure (BP) reduction in patients with hypertension at high CV risk. Treatment with amlodipine led to earlier and more pronounced improvement in BP. There was no difference in the proportion of patients who experienced the primary composite endpoint of cardiac morbidity and mortality (HR, 1.04; 95% CI, 0.94 to 1.15; p=0.49) [Julius S et al. Lancet 2004].
Mortality Benefits of ACE Inhibition in Patients With Coronary Artery Disease
Reproduced from Bertrand ME et al. Clinical synergy of perindopril and calcium-channel blocker in the prevention of cardiac events and mortality in patients with coronary artery disease. Post hoc analysis of the EUROPA study. Am Heart J 2010; 159(5):795–802. With permission from Elsevier.
In the Valsartan in Acute Myocardial Infarction study [VALIANT], valsartan was as effective as the ACE inhibitor captopril in reducing all-cause mortality post MI (HR, 1.00; 95% CI, 0.90 to 1.11; p=0.98), but the combination of the two significantly (p<0.05) increased the rate of adverse events (AEs). There was no improvement in survival (HR for combination therapy versus captopril, 0.98; 95% CI, 0.89 to 1.09; p=0.73). AEs that were significantly higher with combination therapy included hypotension (1.9% of subjects vs 0.8% with captopril monotherapy) and renal dysfunction (1.3% vs 0.8%; both p<0.05) [Pfeffer MA et al. N Engl J Med 2003]. In ONTARGET, the ARB telmisartan was noninferior to the ACE inhibitor ramipril on the primary composite outcome of death from CV causes, MI, stroke, or hospitalization for heart failure in patients with vascular disease or high-risk diabetes (RR, 1.01; 95% CI, 0.94 to 1.09). The combination was associated with an increased risk of hypotension (4.8% vs 1.7%; p<0.001), syncope (0.3% vs 0.2%; p=0.03), and renal dysfunction (13.5% vs 10.2%; p<0.001) but no increase in benefit (RR, 0.99; 95% CI, 0.92 to 1.07) [ONTARGET Investigators. N Engl J Med 2008].
ARBs have also shown benefit in patients with heart failure. In the ELITE II trial the rate of death was similar between losartan and captopril (HR, 1.13; 95% CI, 0.95 to 1.35; p=0.16). Similar frequencies of worsening heart failure (25%) were reported for each group, but losartan was better tolerated, with significantly fewer patients discontinuing treatment because of AEs (p<0.001) [Konstam MA et al. Am Heart J 2005]. In the CHARM study, candesartan was generally well tolerated and significantly reduced CV deaths (18% vs 20%; covariate adjusted HR, 0.87; 95% CI, 0.78 to 0.96; p=0.006) and hospital admissions for heart failure (20% vs 24%; p<0.0001).
Ejection fraction or treatment at baseline did not alter these effects [Pfeffer MA et al. Lancet 2003]. There are also data from the CHARM study indicating that the use of ARBs may be associated with a decreased incidence of diabetes (HR, 0.78; 95% CI, 0.64 to 0.96; p=0.020) [Yusuf S et al. Circulation 2005].
Dr. Elliott noted that ACE inhibition remains the first-line choice for treatment of hypertension, ischemic heart disease, and congestive cardiac failure. Evidence is growing for the efficacy of ARBs but no study to date has shown their superiority over ACE inhibition, which shows definite mortality reduction. Among the ACE inhibitors, only perindopril has solid evidence for mortality reduction.
- © 2013 MD Conference Express®