Summary
Since early studies showing that aspirin reduces the incidence of death and myocardial infarction in patients with unstable angina [Wallentin LC. J Am Coll Cardiol 1991], there has been a search for more effective antithrombotic drugs. The ideal antiplatelet agent should minimize the risk of bleeding risk while maintaining ischemic protection. This article reviews several of the clinical trials that have lead to the paradigm shift in antiplatelet therapy.
- Thrombotic Disorders
- Hemorrhagic Disorders
- Thrombotic Disorders
- Hemorrhagic Disorders
- Hematology
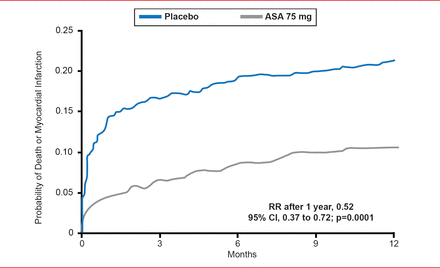
Since early studies showing that aspirin reduces the incidence of death and myocardial infarction (MI) in patients with unstable angina [Wallentin LC. J Am Coll Cardiol 1991], there has been a search for more effective antithrombotic drugs (Figure 1).
Efficacy of Aspirin in Reducing Death or Myocardial Infarction in Patients With Unstable Angina
Reproduced from Wallentin LC. Aspirin (75 mg/day) after an episode of unstable coronary artery disease: Long-term effects on the risk for myocardial infarction, occurrence of severe angina and the need for revascularization. J Am Coll Cardiol 1991;18(7):1581593. With permission from Elsevier.
The ideal antiplatelet agent should minimize the risk of bleeding risk while maintaining ischemic protection. Dominick Angiolillo, MD, PhD, University of Florida College of Medicine, Jacksonville, Florida, USA, reviewed several of the clinical trials that have lead to the paradigm shift in antiplatelet therapy.
Until recently, dual antiplatelet therapy with aspirin and clopidogrel has been the mainstay of secondary prevention of atherothrombotic events in patients with acute coronary syndromes (ACS) or those undergoing percutaneous coronary intervention (PCI). However, treatment-induced adverse bleeding remains a problem. With new stent technology involving more high-risk procedures, more potent platelet inhibition is often needed. In addition to Clopidogrel, the P2Y12ADP receptor antagonists include ticlopidine, prasugrel, and ticagrelor.
In combination with aspirin, ticlopidine reduces the incidence of cardiac and hemorrhagic events and vascular complications in high- and low-risk stented patients when compared with conventional anticoagulants [Schömig A et al. N Engl J Med. 1996; Urban P et al. Circulation 1998]. Ticlopidine, although very effective, has significant side effects (neutropenia, thrombocytopenia) and a delayed timeframe for reaching its full antiplatelet effect. Although Clopidogrel has a better safety profile, faster onset, and better clinical outcomes after stent deployment compared with ticlopidine [Bhatt DL et al. J Am Coll Cardiol 2002], Clopidogrel “resistance” has been described post-PCI and associated with increased platelet reactivity [Angiolillo DJ et al. Am J Cardiol 2006]. Post-stenting prasugrel overcomes this high on-Clopidogrel platelet reactivity more effectively than high-dose Clopidogrel and is approved for clinical use in patients with ACS undergoing PCI [Alexopoulos D et al. JACC Cardiovasc Interv 2011]. The direct acting P2Y12ADP inhibitor, ticagrelor, produces significantly higher platelet inhibition compared with prasugrel in patients with ACS and high on-treatment platelet reactivity while on Clopidogrel 24-hours post-PCI [Alexopoulos D et al. J Am Coll Cardiol 2012].
Prasugrel should not be used in patients with prior stroke or transient ischemic attack or in patients who are being managed medically and at high risk of bleeding, while ticagrelor has applicability in the entire spectrum of ACS, regardless of whether the patient is managed medically or invasively. Ticagrelor should be avoided, however, in patients with high risk of bleeding, prior hemorrhagic stroke, or severe hepatic dysfunction.
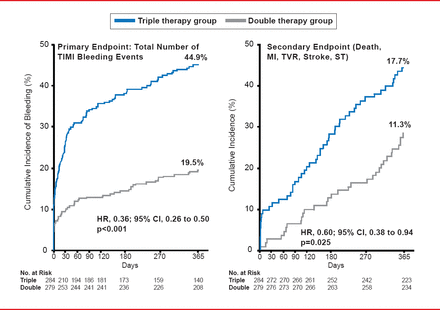
Despite their benefits, increased bleeding has followed increased efficacy with these new agents and since increased bleeding increases the risk of death in the first year after ACS [Mehran R et al. Eur Heart J 2009], there is a need for new strategies to balance safety and efficacy. These strategies include identifying patients at risk for bleeding and ischemia using baseline demographic (eg, advanced age) and clinical characteristics (eg, diabetes mellitus, history of bleeding, and ST-elevation MI) [Moscucci M et al. Eur Heart J 2003]. A major paradigm shift in thinking occurred when the WOEST Trial showed that the use of Clopidogrel without aspirin was associated with a significant reduction in bleeding complications and no increase in the rate of thrombotic events (Figure 2) [Dewilde WJ et al. Lancet 2013]. As a result, several ongoing studies such as PIONEER AF-PCI [NCT01830543], Global Leaders [NCT01813435], and COMPASS [NCT01776424] are reevaluating the role of aspirin in post-PCI therapy
Clopidogrel With and Without Aspirin: The WOEST Study
Reproduced from Dewilde WJM et al. Use of Clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381(9872):1107–115. With permission from Elsevier.
Dosing strategies and newer agents are also being tested. Clinical trials are being conducted with newer agents such as elinogrel (the first oral and intravenous P2Y12 inhibitor), terutroban, vorapaxar, and cangrelor with varying degrees of success.
In one trial, terutroban, a selective thromboxane-prostaglandin receptor antagonist, was found to be no more effective than aspirin [Bousser MG et al. Lancet 2011]. Vorapaxar is a first-in-class oral protease-activated receptor (PAR)-l inhibitor with a long terminal half-life. In patients with ACS, vorapaxar added to standard therapy did not significantly reduce the incidence of death from cardiovascular causes, MI, stroke, recurrent ischemia with rehospitalization, or urgent coronary revascularization, but did increase the risk of bleeding [Tricoci P et al. N Engl J Med 2012]. In another study, although it did reduce the risk of cardiovascular death or ischemic events in patients with stable atherosclerosis receiving standard therapy, vorapaxar was again associated with increased moderate or severe bleeding, including intracranial hemorrhage [Morrow DA et al. N Engl J Med 2012].
Cangrelor is a direct platelet P2Y12 antagonist characterized by rapid, potent, predictable, and reversible platelet inhibition. It may be the ideal drug for immediate platelet inhibition, a feature particularly desirable in high-risk settings such as patients with ST-elevation MI undergoing primary PCI. Although cangrelor was not superior to Clopidogrel in reducing ischemic events in the CHAMPION trial [Angiolillo DJ et al. J Thromb Thrombolysis 2012], the PHOENIX trial reported that cangrelor was significantly better (p=0.005) compared with Clopidogrel at reducing ischemic events during PCI [Bhatt DL et al. N Engl J Med 2013].
Minimizing the risk of bleeding while maintaining ischemic protection is an important but often elusive endpoint in clinical trials of antiplatelet drugs. Rivaroxaban is a factor Xa inhibitor. In a double-blind, placebo-controlled trial in patients with ACS, rivaroxaban significantly reduced the risk of the composite endpoint (cardiovascular death, MI, stroke), cardiovascular death, and all-cause death. However, risk of major bleeding, but not fatal bleeding was also increased [Mega JL et al. N Engl J Med 2012].
Antiplatelet therapy is important for preventing atherothrombotic events in patients with coronary disease, but finding the “sweet spot” between a high risk of ischemic events and a high risk of bleeding events is the ideal goal. The benefit of adding novel oral anticoagulants (ie, rivaroxaban) to dual antiplatelet therapy and the safety of discontinuing aspirin after a short term of dual antiplatelet therapy with prasugrel or ticagrelor warrant further investigation.
- © 2013 MD Conference Express®