Summary
Although the guidelines for treating venous thromboembolism (VTE) in cancer patients recommend anticoagulation with low-molecular-weight heparin for at least 3 to 6 months, or for the duration of the malignancy, uncertainty exists concerning whether to extend anticoagulation longer to prevent recurrence of VTE. The objective of the Evaluation of Dalteparin for Long-Term (One Year) Treatment of Blood Clots in Subjects With Cancer study [DALTECAN; NCT00942968] was to assess the consequences of extending anticoagulation with dalteparin beyond 6 months in cancer-associated VTE.
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
- Hematology Clinical Trials
- Thrombotic Disorders
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
- Hematology Clinical Trials
- Hematology
- Thrombotic Disorders
Although the guidelines for treating venous thromboembolism (VTE) in cancer patients recommend anticoagulation with low-molecular-weight heparin for at least 3 to 6 months, or for the duration of the malignancy, uncertainty exists concerning whether to extend anticoagulation longer to prevent recurrence of VTE. In a study presented by The Lord Ajay K. Kakkar, MD, University College London, London, United Kingdom, dalteparin sodium administered beyond 6 months was not associated with an increase in bleeding relative to the initial period of therapy.
The objective of the Evaluation of Dalteparin for Long-Term (One Year) Treatment of Blood Clots in Subjects With Cancer study [DALTECAN; NCT00942968] was to assess the consequences of extending anticoagulation with dalteparin beyond 6 months in cancer-associated VTE. This was a Phase 4 multicenter, single-arm, open-label study that enrolled men and women aged ≥18 years with histologically confirmed cancer and a new VTE (objectively confirmed deep vein thrombosis [DVT], proximal vein thrombosis, pulmonary embolism, or both). All patients received treatment with dalteparin 200 IU/kg daily subcutaneously for 1 month, followed by 150 IU/kg daily for up to 11 months. The study was conducted at 50 sites in the United States, Europe, and Canada.
The primary study endpoint was major bleeding events for Months 7 to 12 relative to Months 2 to 6 of dalteparin therapy. Secondary endpoints included rate of recurrent VTE, time to symptomatic recurrent VTE, rate of minor bleeding events, time to first major or any bleeding event, and safety and tolerability of extended treatment with dalteparin. Bleeding and recurrent VTE were centrally adjudicated.
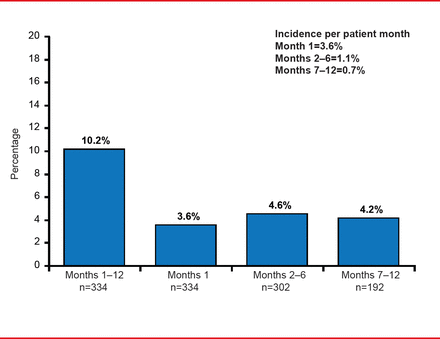
Patients had a mean age of 64 years and about half were men. Most (78%) were Eastern Cooperative Oncology Group (ECOG) Performance Status 0 or 1. Of the 334 patients who received drug treatment, 109 (32.2%) completed the 12-month study. Of the 229 patients who discontinued the study, 76 were due to death and 60 were due to an adverse event. The remaining discontinuations were due to withdrawal of consent (n=42) or “other” reasons (n=51). Most (92%) patients had solid tumors and the majority was stage III or IV Therapy adherence was 96% across the entire cohort, with a median treatment duration of 211 days. The overall frequency of major bleeding was 10.2%, observed at a rate of 1.3% per patient-month.
The Incidence of Major Bleeding Events
The highest major bleeding rate was in the first month of dalteparin therapy at 3.6%, with the frequency declining to 1.1% during Months 2 to 6, and 0.7% over Months 7 to 12, with no statistically significant difference in rates between Months 2 to 6 and 7 to 12 (p=0.39). The incidence of all bleeding events was 13.2% in Month 1, 4.5% in Months 2 to 6, and 2.7% in Months 7 to 12, with a total 1 to 12 month incidence rate of 33.5%.
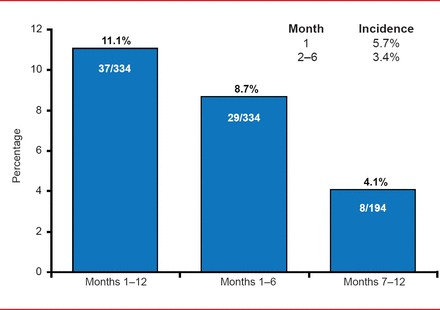
The incidence of new or recurrent VTE was 11.1% (37 patients), a rate of 1.4% per patient-month. The rate was highest for Month 1 at 5.7%, falling thereafter to 0.8% per month for Months 2 to 6 (incidence 3.4%) and 0.7% per month for Months 7 to 12 (incidence 4.1%). The investigators were unable to identify any predictive factors for the occurrence of VTE.
Incidence of New or Recurrent VTE
Extending dalteparin was not associated with an increase in major bleeding and adherence to therapy was high, leading the investigators to conclude that it is feasible to extend dalteparin therapy beyond 6 months in patients with cancer.
- © 2013 MD Conference Express®