Summary
The European Heart Rhythm Association (EHRA) scientific documents provide advice based on scientific data in emerging areas of arrhythmia management in Europe. The documents contain advice rather than guidelines because insufficient evidence exists to develop specific recommendations in these areas. This article discusses left atrial appendage occlusion for atrial fibrillation, practical advice when using new oral anticoagulants, heart imaging in patients undergoing ablation, electrophysiological procedures in children, and a health technology assessment for electrophysiological procedures.
- Interventional Radiology
- Interventional Techniques & Devices
- Imaging Modalities
- Arrhythmias
- Interventional Radiology
- Cardiology & Cardiovascular Medicine
- Interventional Techniques & Devices
- Imaging Modalities
- Arrhythmias
The European Heart Rhythm Association (EHRA) scientific documents provide advice based on scientific data in emerging areas of arrhythmia management in Europe. The documents contain advice rather than guidelines because insufficient evidence exists to develop specific recommendations in these areas.
LEFT ATRIAL APPENDAGE OCCLUSION FOR ATRIAL FIBRILLATION
Michael Glikson, MD, Chaim Sheba Medical Center, Tel Hashomer, Israel, presented draft findings of the EHRA and European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Document Committee on the European Cardiac Resynchronization Therapy Survey: comparison of outcomes between de novo cardiac resynchronization therapy implantations and upgrades (LAAO) for atrial fibrillation (AF).
Table 1 summarizes the committee's findings on the standards and requirements, devices, imaging, and data collection and registries for LAAO in patients with AF.
Oral anticoagulants remain the standard for first-line treatment of AF. New oral anticoagulants (NOACs) are preferred for patients with excessive risk of anticoagulation. LAAO has not been compared with NOACs in clinical trials. However, the risk of bleeding with some NOACs, including low dose dabigatran and apixaban, is expected to be lower than with warfarin.
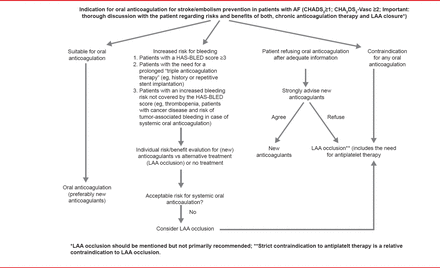
LAAO should be considered for patients with an unacceptable risk of bleeding with oral anticoagulation, who refuse oral anticoagulation, or those with contraindications for any oral anticoagulation (Figure 1). The committee found no evidence to support LAAO as an adjunct to oral anticoagulants or AF ablation. Prof. Glikson concluded that future randomized controlled trials are needed to provide evidence for the use of LAAO in patients with AF, particularly to study the Amplatzer Cardiac Plug device, high-risk populations with contraindications to oral anticoagulants, LAAO versus NOACs, future devices, and epicardial approaches.
Indications for Left Atrial Appendage Occlusion
Scientific Document Committee Findings for LAAO for AF
NEW ORAL ANTICOAGULANTS – PRACTICAL ADVICE FOR DIFFICULT SITUATIONS
The EHRA has developed a practical guide for the use of NOACs in patients with nonvalvular AF [Heidbuchel H et al. Europace 2013]. Lead author, Hein Heidbuchel, MD, PhD, University of Leuven, Leuven, Belgium, said that the intent of the guide is to inform physicians on how to use these drugs safely and effectively in clinical practice. The guide addresses the four NOACs: dabigatran, rivaroxaban, apixaban, and edoxaban. All but edoxaban are approved by the European Medicines Agency; edoxaban currently is under investigation in the ENGAGE-AF trial [NCT00781391], with results expected at the American Heart Association (AHA) 2013 meeting. An associated Web site, www.NOACforAF.eu, complements the published guide, including patient anticoagulation cards, feedback forms, a slide kit, a key messages pocket guide, and updates.
The EHRA guide provides a practical start-up and follow-up scheme for physicians to use when prescribing NOACs. Detailed information on absorption and metabolism of NOACs is provided (Table 2) as well as an overview of potential drug-drug interactions and comorbidities which may require dosing considerations. The guide also discusses on how to manage dosing errors.
Absorption and Metabolism of NOACs
The guide includes detailed tables on cessation before planned and urgent surgery, bleeding complications, and use of coagulation assays with the NOACs.
The use of NOACs in patients with AF and coronary artery disease, kidney disease, and those undergoing ablation or cardioversion, as well as management of patients with acute stroke or acute coronary syndrome while on NOACs are also addressed. Although scientific evidence is lacking for some of these issues, updated information will be provided on the EHRA website (www.NOACforAF.eu) as data become available.
HEART IMAGING IN PATIENTS UNDERGOING ABLATION
The EHRA consensus statement on imaging for electrophysiological and device procedures was developed at the first EHRA Policy Conference [Lundqvist CB et al. Europace 2013]. According to lead author Carina Blomström Lundqvist, MD, PhD, Uppsala University, Uppsala, Sweden, the objective of the Policy Conference was to assess the state of evidence and possibility for formal recommendations on the development and use of new imaging tools in electrophysiology and device implantation (Table 3).
Imaging Requirements and Uses
The Policy Conference brought together experts in the areas of radiology, electrophysiology, and cardiac imaging to assess the evidence and develop a guide for heart imaging in patients undergoing ablation. The resulting document is a key step in guiding the future clinical use of these technologies.
ELECTROPHYSIOLOGICAL PROCEDURES IN CHILDREN
Josep Brugada Terradellas, MD, PhD, University of Barcelona, Barcelona, Spain, presented the EHRA/Association for European Pediatric Cardiology (AEPC) consensus document titled Pharmacological and Non-Pharmacological Therapy for Arrhythmias in the Pediatric Population, focusing on electrophysiological procedures in children [Not yet published].
Device implantation in children is a challenge, requiring the use of both the transvenous and epicardial routes. The technique used depends on the size of the patient, underlying anatomy, venous patency, and experience of the unit. Complications are more frequent in children than in adults.
According to Prof. Brugada, pediatric catheter ablation should be performed in specialized centers by electrophysiologists experienced in pediatric ablations in collaboration with pediatric cardiologists. Three-dimensional mapping and nonfluoroscopic navigation is required for ablation of complex arrhythmias such as those associated with congenital heart disease. While ablation can be performed in older children under conscious or deep sedation, most patients aged <10 years require general anesthesia. Modifications of the ablation procedure may be needed in small children to avoid complications, including use of 5F radiofrequency ablation catheters and single diagnostic electrophysiological catheters. Lower power and temperature settings may reduce the risk of coronary and myocardial injury. Indications for pediatric catheter ablation are shown in Table 4.
Prof. Brugada emphasized that individual decision making is of “utmost importance,” especially in young children. Antiarrhythmic drug therapy can be effective and may be preferable in certain situations, particularly in younger children.
Indications for Pediatric Catheter Ablation
HEALTH TECHNOLOGY ASSESSMENT FOR ELECTROPHYSIOLOGICAL PROCEDURES
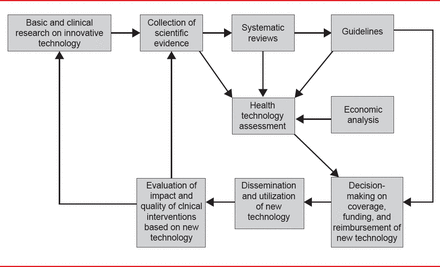
The Health Technology Assessment in Interventional Electrophysiology and Device Therapy: A Position Paper of the EHRA [Boriani G et al. Eur Heart J 2013] was presented by Giuseppe Boriani, MD, PhD, University of Bologna, Bologna, Italy. Health technology assessments (HTAs) are conducted by a multidisciplinary team to evaluate the clinical- and cost-effectiveness of healthcare interventions. Factors considered in an HTA include quality, safety, efficacy, cost, cost-effectiveness, and organizational, legal, and social aspects. The EHRA HTA process is outlined in Figure 2.
Health Technology Assessment Process
Reproduced from Boriani G et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34(25):1869–1874. With permission from Oxford University Press.
The benefits of electrophysiological interventions include prolonging life, preventing cardiovascular death and stroke, improving exercise capacity and quality of life, preventing symptoms of arrhythmia and hospitalizations, and improving social and cognitive functioning. However, economic pressures may limit availability of devices with a high upfront cost, such as implantable cardioverter-defibrillators. HTAs employ an economic analysis, including cost-effectiveness, cost-utility, and cost-benefit, rather than a financial analysis that considers only cost.
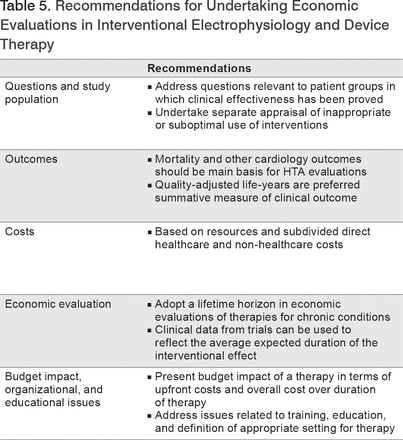
The EHRA recommendations for HTAs in interventional electrophysiology and device therapy are summarized in Table 5.
Recommendations for Undertaking Economic Evaluations in Interventional Electrophysiology and Device Therapy
Prof. Boriani cautioned against getting caught in the “innovation paradox,” in which older or cheaper products are bought to cut costs, resulting in reduced quality and service, and ultimately, a decline in productivity and efficiency. Instead it is important to embrace innovation by investing in optimizing healthcare delivery to patients to achieve health outcomes and quality goals, resulting in improved productivity and efficiency.
The editors would like to thank the many members of the EHRA EUROPACE presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2013 MD Conference Express®