Summary
Due to multiple challenges of the current technology of catheter ablation therapy of atrial fibrillation (AF), new technologies have been developed. This article discusses the effect of balloon technologies in ablation of AF, the use of advanced radiofrequency ablation in AF, the challenge of ventricular tachycardia ablation, as well as potential advantages of robotic catheter navigation in complex arrhythmias.
- Arrhythmias
- Interventional Radiology
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
Due to multiple challenges of the current technology of catheter ablation therapy of atrial fibrillation (AF), new technologies have been developed. Marc Dubuc, MD, Université de Montreal, Montreal, Quebec, Canada, discussed the effect of balloon technologies in ablation of AF. Currently, there are three balloon technologies available for ablation: high-intensity focused ultrasound (HIFU), laser balloon technology, and cryoballoon. HIFU is no longer used due to safety concerns.
The laser balloon technology uses a compliant balloon with direct visualization of the pulmonary vein ostium. Prof. Dubac pointed out that it is not a “single-shot” technology. Based on data from a total of 406 patients worldwide, the efficacy and safety of laser balloon technology is comparable to other balloon technologies. Results from a clinical trial evaluating laser balloon technology in 450 patients with AF are anticipated to be released in May 2014.
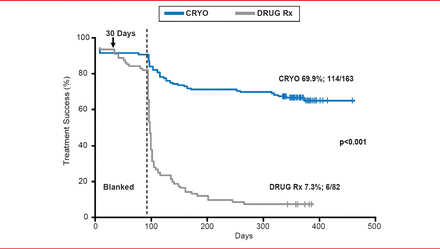
In contrast, cryoballoon ablation has been performed in over 55,000 patients with AF worldwide. The cryoballoon catheter consists of double polyurethane and polyester balloons and does not require 3D mapping. Prof. Dubac pointed out that cryoballoon ablation is a “single-shot” technology, unlike the laser balloon. In a prospective, multicenter study of 346 patients with AF, 97% of pulmonary veins were isolated with the 23- or 28-mm balloon, while only 9.5% of patients required >1 size of balloon [Neumann T et al. J Am Coll Cardiol 2008]. Following ablation with the cryoballoon, 74% of paroxysmal AF patients and 42% of persistent AF patients were free of AF. Adverse events included 2 tamponades, 3 atrioventricular fistula or pseudoaneurysms, and 26 patients experienced right phrenic nerve paralysis. In the STOP-AF pivotal trial [Packer DL et al. J Am Coll Cardiol 2013], treatment success was achieved in 69.9% of AF patients, compared with 7.3% in patients that received pharmacologic therapy (p<0.001; Figure 1). A systemic review of published studies of cryoballoon safety and efficacy reported the chronic success rate to be 72.8% (95% CI, 68.79% to 76.62%) [Andrade JG et al. Heart Rhythm 2011].
Treatment Success Following Abalation by Cryoballoon
Reproduced from Packer DL et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American arctic front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61(16):1713–1723. With permission from Elsevier.
Erik Wissner, MD, Asklepios Klinik St. Georg, Hamburg, Germany, presented information about advanced radiofrequency ablation (RFA) in AF, particularly the importance of contact force. The goal in RFA is to create sufficient lesion depth, while minimizing complications. Catheter contact force is important in determining the depth and size of the lesion. A greater contact force requires a lower RF to produce a lesion of the same quality [Thiagalingam A et al. J Cardiovasc Electrophysiol 2010]. Prof. Wissner pointed out that the key challenge in RFA is force control, as insufficient contact can result in a lengthy procedure that may need to be repeated at a later date, and excessive force carries a greater risk of complications such as tamponade, esophageal injury, and steam pops.
A prospective, single-arm trial has been conducted to determine the efficacy of using force sensing during RFA procedures. Results from the EFFICAS I study demonstrated that an ideal contact force is about 20 g [Neuzil P et al. Circ Arrhythm Electrophysiol 2013]. Other recommendations based on EFFICAS I data include avoiding RF use if contact force is <10 g; targeting a force-time integral of 600 g x seconds; and avoiding early termination of RF application, which may result in edema formation rather then lesion transmurality.
Prof. Wissner highlighted data from a porcine model, in which the minimum perforation force with RFA was determined to be 131 g in the right atrium, 159 g in the left atrium, 168 g in the right ventricle, and 227 g in the left ventricle [Shah D et al. Europace 2011]. However, in another study of living porcine atria, the lowest perforating contact force was 77 g [Perna F et al. Circ Arrhythm Electrophysiol 2011]. Importantly, perforation at lower contact force is more likely at sites previously targeted for ablation.
John Sapp, MD, QEII Health Sciences Centre, Halifax, Nova Scotia, Canada, discussed the challenge of ventricular tachycardia ablation, despite several new technological advances. Prof. Sapp pointed out that one of the major limitations in ablation is insufficient lesion size/depth. Some factors important in lesion size include RF duration, power, electrode diameter and contact area, contact pressure, and impedance.
Prof. Sapp suggested that one way to bring the electrode closer to the tissue is by needle ablation, whereby a retractable needle that can penetrate the tissue is located at the tip of the catheter. Infusion needle ablation increases the conductivity of the tissue and permits deep lesion creation. Prof. Sapp presented data from 8 patients with ventricular tachycardia (VT) that had failed multiple pharmacologic interventions and previous ablations. The patients were treated with needle ablation. During the follow-up period of 12 months, 4 of the 8 patients were free of recurrent VT, 2 patients developed new VT that were treated with pharmacologic therapy, and 2 patients had frequent recurrences. Adverse events included 2 heart blocks and 1 tamponade.
Jose L. Merino, MD, Hospital Universitario La Paz, Madrid, Spain, presented potential advantages of robotic catheter navigation in complex arrhythmias. Some of the problems with conventional, manually guided ablation are that the catheter is difficult to navigate to certain sites and maintain catheter stability while making tissue contact, as well as safety concerns such as excessive contact pressure. These challeges could be overcome by robotic catheter navigation, especially with the magnetic systems.
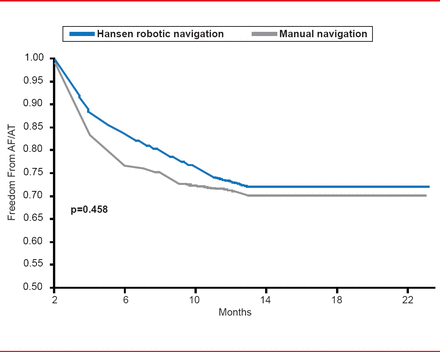
However, a recent study demonstrated similar rates of freedom from AF following robotic navigated ablation compared with manual navigation (Figure 2) [Di Biase L et al. J Cardiovasc Electrophysiol 2009]. Currently, there are two types of remote navigation systems, mechanically guided (or true robotic ablation) and magnetically guided ablation. Although further studies are needed to determine safety and efficacy, magnetically guided ablation appears to increase tissue contact and stability, is convenient, and appears to be effective in complex cases. However, there is a learning curve for the physician and present results came from the first generations of these systems.
Robotic and Manual Navigation of Ablation in AF
Reproduced from Di Biase L et al. Ablation of Atrial Fibrillation Utilizing Robotic Catheter Navigation in Comparison to Manual Navigation and Ablation: Single-Center Experience. J Cardiovasc Electrophysiol 2009;20(12):1328–1335.
There are multiple new technologies available for ablation therapy of AF that potentially offer improved safety and efficacy. Although further research is required for many of the new technologies, promising results have been demonstrated for balloon catheters, RFA, needle ablation, and robotic-guided ablation.
- © 2013 MD Conference Express®