Summary
Hypertension (HTN) affects more than 25% of adults in developed societies [Wolf-Maier K et al. Hypertension 2004] and is a leading attributable cause of mortality worldwide, causing 7.5 million deaths annually [Symplicity HTN-1 Investigators. Hypertension 2011]. Refractory or resistant hypertension, which is defined as elevated blood pressure despite full doses of 3 antihypertensive agents, including a diuretic, is being increasingly recognized as a clinically important problem that might affect 13% of the hypertensive population [Geisler BP et al. J Am Coll Cardiol 2012]. Recently, catheter-based renal denervation treatment has proven to be a viable therapeutic approach for resistant HTN [Geisler BP et al. J Am Coll Cardiol 2012]. This article discusses emerging technologies and new renal denervation devices in development.
- Hypertensive Disease
- Cardiology
Hypertension (HTN) affects more than 25% of adults in developed societies [Wolf-Maier K et al. Hypertension 2004] and is a leading attributable cause of mortality worldwide, causing 7.5 million deaths annually [Symplicity HTN-1 Investigators. Hypertension 2011]. Every 20/10 mm Hg increase in blood pressure (BP) is associated with a doubling of cardiovascular mortality [Lewington S et al. Lancet 2002; Chobanian AV et al. Hypertension 2003]. Refractory or resistant hypertension, which is defined as elevated BP despite full doses of 3 antihypertensive agents, including a diuretic, is being increasingly recognized as a clinically important problem that might affect 13% of the hypertensive population [Geisler BP et al. J Am Coll Cardiol 2012].
Recently, catheter-based renal denervation treatment has proven to be a viable therapeutic approach for resistant HTN [Geisler BP et al. J Am Coll Cardiol 2012]. Horst Sievert, MD, PhD, Johann Wolfgang Goethe University Frankfurt, Frankfurt, Germany, discussed emerging technologies and new renal denervation devices in development.
A revolutionary treatment principle, renal denervation uses ablation of the renal sympathetic nerves with a radiofrequency (RF)-emitting catheter inserted percutaneously via the femoral artery into the lumen of both renal arteries [Laurent S et al. Lancet 2012]. The randomized controlled Symplicity HTN-2 trial confirmed a systolic BP (SBP) reduction of 32±23 mm Hg compared with a change of +1±21 mm Hg observed for standard of care (p<0.0001) from a baseline SBP of 178±17 mm Hg [Esler MD et al. Lancet 2010].
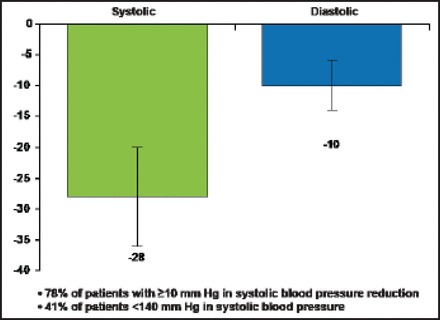
A multielectrode and RF-ablation–based system, EnligHTN, lowered patients' BP even faster. Thirty-day results in 47 resistant hypertension patients showed mean office BP changes at 1 month of −28 mm Hg systolic and −10 mm Hg diastolic (p<0.0001 from baseline), with 78% of patients having systolic BP drops of ≥10 mm Hg and 41% having SBP <140 mm Hg (Figure 1) [Worthley S et al. EuroPCR 2012]. In the ARSENAL study [NCT01438229] of the EnligHTN system, no serious complications were seen in the renal artery or at the access site; minor procedure-related events included 4 hematomas, 3 vasovagal responses to sheath removal, and 2 postprocedure transient bradycardias.
30-Day Results from St. Jude's Medical First-in-Man Study on the Multielectrode and RF-Ablation-Based EnligHTN System.
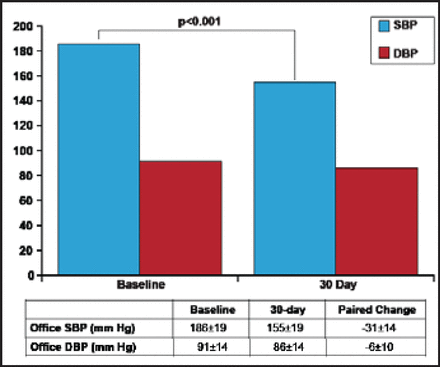
The renal denervation system OneShot received European CE mark clearance in February 2012 [Valigra L. Mass High Tech 2012]. Patients in its first-in-man [FIM] study (n=9) had baseline office SBP of 186±18 mm Hg and diastolic BP (DBP) of 91±14 mm Hg. The decrease in office SBP (155±19 mm Hg) and DBP (86±14 mm Hg) at 30 days was statistically significant (p<0.001; Figure 2) [Ormiston J et al. EuroPCR 2012]. The balloon was inflated to 1 atm for 2 minutes ablation of each side; irrigation cooling protected the nontreated region of the artery. Technical success was achieved in 8 of 9 patients. The only failure required minor reprogramming of the RF generator. The median total procedure time was 34 minutes; median fluoroscopy time was 8 minutes; median contrast volume 126 mL. No adverse events were reported.
30-Day Outcomes from Covidien's OneShot Renal Denervation System.
DBP=diastolic blood pressure; SBP=systolic blood pressure.
The Vessix V2 renal denervation system also has received European CE Mark approval. This system uses a balloon catheter with bipolar RF electrodes, low pressure (<3 atm), and simultaneous energy delivery to all electrodes. Suitable for renal arteries of 3 to 7 mm, treatment time is 30 seconds using <1/2 to 1 watt. In the Vessix FIM trial (n=7), 30-day results demonstrated declines in SBP of −28 mm Hg and in DBP of −11 mm Hg (95% CI) [REDUCE-HTN pilot clinical trial. EuroPCR 2012]. The response rate (defined as a change in SBP and DBP) was 100%. This compared to a 1 month −19 mm Hg drop in SBP and a −9 mm Hg decline in DBP seen in the Symplicity HTN-1 trial (n=138). A multicenter, international Vessix study is currently underway [REDUCE-HTN; NCT01541865]. It is the first clinical trial to treat patients with accessory renal arteries.
Looking to the Future
According to Prof. Sievert, likely areas for future innovation include novel drugs, ultrasound, sound intervention, and radiation.
Nano particles are being developed (approximately 100 nanometers) to treat refractory hypertension. Biodegradable catheters have a paramagnetic core with polymeric coating and Botox B. The particles are injected into the renal artery and pulled inside the artery wall by a magnetic field. Two possible mechanisms include heat generated by the magnetic field or Botox released by the particles.
A catheter tipped with a balloon-sheathed microneedle is another innovation. It is guided and inflated in a manner similar to an angioplasty catheter but with far lower expansion pressures (2 atm). The catheter deploys a microneedle into the adventitia, allowing drug delivery to the renal sympathetic nerve sheath. Catheters are available for >2 mm arteries.
A nonfocused ultrasound system for performing renal denervation in patients with resistant hypertension consists of an ultrasound transducer mounted inside a 6 F balloon catheter. The ultrasound creates heat within the surrounding structures and tissue, while cooled water in the balloon protects the endothelium against heat.
Other technologies under development include the Cardiosonic TIVUS System, with outcomes from a FIM trial expected in 2013, sound intervention, and radiation. Sound intervention does not require wall contact, and uses dosimetry-based “targeting” of nerves with 360 degrees of unfocused ultrasound and blood flow to keep the artery cool. The Beta-Cath 3.5F System delivers β-radiation, which offers potential “sparing” of endothelial injury, a localized effect, small sheath size, and a short procedure time of 5 to 8 minutes per artery [Waksman R. EuroPCR 2012].
- © 2012 MD Conference Express®