Summary
This article presents findings from a single center experience with the WATCHMAN left atrial appendage (LAA) closure.
- Arrhythmias
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology
Samih Lawand, MD, King Fahad Medical City, Riyadh, Saudi Arabia, presented findings from a single center experience with the WATCHMAN left atrial appendage (LAA) closure.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. Estimated to afflict >5.5 million people in the United States alone, the number is expected to increase to >15 million by the year 2050 [Reddy VY et al. Circulation 2011].
In patients with AF, stroke is the number 1 cause of long-term disability and the third leading cause of death [Savelieva I et al. Ann Med 2007]. The rhythm increases a patient's risk of an ischemic stroke by 4- to 5-fold, and accounts for up to 20% of all ischemic strokes [Magnani JW et al. Circulation 2011].
Ischemic strokes occur in 5% of non-anticoagulated AF patients each year [Crandall MA et al. Mayo Clin Proc 2009]. While anticoagulation with warfarin reduces the risk of stroke by about 60%, a large proportion of patients with AF do not receive this treatment due to relative/absolute contraindications. Patients also discontinue treatment for a variety of reasons, and long-term warfarin administration rates remain suboptimal [Landmesser U, Holmes DR Jr. Eur Heart J 2012].
Transesophageal echocardiographic (TEE) studies show that 90% of thrombi come from the LAA in nonvalvular AF [Landmesser U, Holmes DR Jr. Eur Heart J 2012]. Transcatheter LAA closure with the WATCHMAN device has become one of the therapeutic options for AF patients who are at high risk for ischemic stroke [Bai R et al. J Cardiovasc Electrophysiol 2012].
The WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation [PROTECT AF; NCT00129545] trial found that the efficacy of percutaneous closure of the LAA with the device was noninferior to warfarin therapy, but that there was a higher rate of adverse safety events in the intervention group than in the control group—mainly periprocedural complications [Holmes DR et al. Lancet 2009].
At the Prince Salman Heart Center, a total of 58 patients underwent LAA occluder device implants. The implantation success rate was 78% (45 patients). After TEE, 8 patients (14%) were found unsuitable for surgery (too small or too large). Unsuccessful implantations requiring device recapture occurred in 3 patients (5%). The procedure was aborted in 1 patient due to an inability to cross the interatrial septum; another was aborted when a left atrial thrombus developed during the procedure. LAA perforation requiring cardiac surgery occurred in 1 patient. There were no periprocedural deaths.
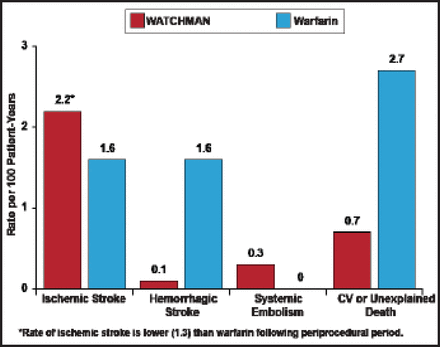
Outcomes were in line with those from the PROTECT AF trial: LAA device closure (in AF patients who were candidates for warfarin) was associated with a reduction in the rate of hemorrhagic stroke risk versus warfarin. Rates of all-cause stroke and all-cause mortality were noninferior to warfarin (Figure 1), whereas safety events, primarily pericardial effusion with or without tamponade, were more common in the device group.
WATCHMAN Rates of All-Cause Stroke and All-Cause Mortality Were Noninferior to Warfarin in the PROTECT AF Trial.
CV=cardiovascular; PROTECT AF=WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation.
Prof. Lawand concluded that closure of LAA might provide an alternative strategy to long-term warfarin therapy for stroke prophylaxis in patients with nonvalvular AF.
The editors would like to thank the many members of the CardioAlex 2012 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®