Summary
Studies have shown that drug-eluting stents (DES) are associated with delayed arterial healing and are prone to thrombosis. This article discusses the utility of intravascular ultrasound to determine mechanisms of late and very late stent thrombosis after DES implantation.
- Interventional Techniques & Devices
- Thrombotic Disorders
Studies have shown that drug-eluting stents (DES) are associated with delayed arterial healing and are prone to thrombosis. Fausto J. Pinto, MD, PhD, Lisbon University, Lisbon, Portugal, discussed the utility of intravascular ultrasound (IVUS) to determine mechanisms of late and very late stent thrombosis (ST) after DES implantation.
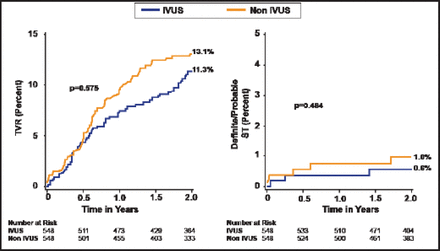
Armstrong et al. [JACC Cardiovasc Interv 2012] evaluated 7079 patients with angiographically documented early (<1 month), late (1 to 12 months), and very late (≥12 months) ST. In-hospital mortality was highest in patients with early ST compared with those who had late or very late ST. Claessen et al. [JACC Cardiovasc Interv 2011] found that IVUS-guided versus angiography-guided DES implantation appeared to be associated with a reduction in early and long-term cardiovascular events (Figure 1).
Clinical Outcomes to 2-Year Follow-Up in the Propensity-Matched Cohort.
IVUS=intravascular ultrasound; TVR=target vessel revascularization.
Reproduced with permission from Elsevier. Claessen B et al. Impact of Intravascular Ultrasound Imaging on Early and Late Clinical Outcomes Following Percutaneous Coronary Intervention With Drug-Eluting Stents. JACC Intervention 2011;4(9):974.
Mechanisms of ST
Mechanisms of ST include edge dissections, stent underexpansion, incomplete stent apposition (ISA), incomplete lesion coverage, geographic miss, tissue protrusion, and residual thrombus. IVUS-guided percutaneous coronary intervention with DES is probably most useful for restenosis, edge dissections, ST, stent underexpansion, and ISA. A recent study [Kang SJ et al. Circ Cardiovasc Interv 2011] using IVUS to study in-stent restenosis after DES found that the primary mechanism was intimal hyperplasia and that underexpansion associated with longer stent length was an important preventable mechanism of ST. Studies have shown that the incidence of persistent edge dissections by IVUS after DES implantation is approximately 10%, of which almost 40% are not detectable by angiography.
Data analysis from multiple large trials shows that potential mechanisms of ST after DES implantation include delayed healing (failure of new neointimal formation and smooth muscle cell replication, poor endothelialization) and clopidogrel-related issues (premature discontinuation, inadequate platelet inhibition). In a Kaplan-Meier analysis, Roy et al. [Eur Heart J 2008] demonstrated significantly longer ST-free survival in patients with IVUS-guided stents versus no IVUS (p=0.013). A HORIZONS substudy [Choi SY et al. Circ Cardiovasc Interv 2011] reported that minimum lumen cross-sectional area (CSA), significant tissue protrusion, significant stent edge dissection, and significant residual stenosis but not acute malapposition were associated with early ST. However, Cook et al. [Eur Heart J 2012] found that the presence of CSA, assessed by IVUS 8 months after DES implantation, was associated with a higher rate of MI and very late ST during long-term follow-up.
The underlying mechanisms of late ST after DES implantation include abnormal or delayed healing, inadequate endothelial cell coverage on struts, and positive remodeling consistent with underlying vascular toxicity. IVUS may help us understand the mechanisms of late ST and reduce the incidence of ST.
- © 2012 MD Conference Express®