Summary
In an update on systemic lupus erythematous, this article provides an overview of the classification and available treatments for variants of cutaneous lupus erythematous. Also discussed are the induction and maintenance therapy for lupus nephritis, as well as other treatments to induce and maintain improvement and prevent further damage for lupus patients.
- Lupus
- Systemic Connective Tissue Disorders
In an update on systemic lupus erythematous (SLE), David Fiorentino, MD, PhD, Stanford University School of Medicine, Stanford, California, USA, provided an overview of the classification and available treatments for variants of cutaneous lupus erythematous (CLE).
Recent studies have shown the incidence of CLE to be similar to that of SLE [Durosaro O et al. Arch Dermatol 2009] with discoid lupus erythematosus (DLE) representing about 70% to 80% of the total, followed by subacute CLE (SCLE; 15% to 20%), and acute CLE (ACLE; 5%) [Grönhagen CM et al. Br J Dermatol 2011]. The spectrum of CLE includes both nonspecific (vasculitis, alopecia, neutrophilic dermatosis) and specific (most examples showing interface dermatitis on biopsy) disease. Among the nonspecific diseases, the most common type of vasculitis is small vessel disease, which is associated with cytopenias and higher disease activity [Ramos-Casals M et al. Medicine 2006; Burling F et al. Lupus 2007]. Other nonspecific signs include alopecia (indicative of active disease) and the neutrophilic disorders, which are less well known.
Generally, the presence of nonspecific symptoms indicates a higher risk of having systemic disease or, in patients with systemic disease, an impending flare. Lupus-specific skin disease includes ACLE, SCLE, and chronic CLE (CCLE). Characterized by a “butterfly rash,” ACLE is strongly associated with SLE (40% to 60% of patients). SCLE can be drug induced (∼20% to 33% of cases) [Lowe G et al. Br J Dermatol 2011; Grönhagen CM et al. Br J Dermatol 2012] and ∼50% meet the criteria for SLE. Most CCLE is DLE [Grönhagen CM et al. Br J Dermatol 2011], and recent data suggest that about 20% of DLE patients will progress to SLE [Parodi A et al. Br J Dermatol 2000], while ∼20% to 30% of SLE patients will eventually get DLE lesions [Sanchez E et al. Ann Rheum Dis 2011; Järvinen TM et al. PLoS One 2010].
Initial treatment for CLE is nonpharmacological (eg, sun protection and smoking cessation). Local therapies beginning with corticosteroids (topical and intralesional), calcineurin inhibitors, and possibly retinoids are recommended. Antimalarials are considered the first-line systemic therapy [Rusicka T et al. Br J Dermatol 1992]. Not all patients respond to hydroxychloroquine as baseline lupus severity and the presence of SLE can influence the response to this agent [Wahie S et al. J Invest Dermatol 2011]. Outcomes can be improved by monitoring hydroxychloroquine blood concentrations [Frances C et al. Arch Dermatol 2012] and the addition of quinacrine [Change AY et al. Arch Dermatol 2011]. Methotrexate is often considered the first-line treatment for antimalarial-resistant CLE. However, good responses have been achieved with the antibacterial dapsone [Coburn PR, Shuster S. Br J Dermatol 1982], the immunomodulatory drug mycophenolate mofetil (MMF) [Kreuter A et al. Br J Dermatol 2007], and the antiangiogenic drug thalidomide [Cortés-Hernández J et al. Br J Dermatol 2012]. There has also been some success with laser surgery.
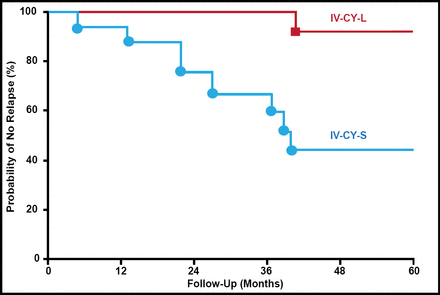
James E. Balow, MD, National Institutes of Health, Bethesda, Maryland, USA, discussed induction and maintenance therapy for lupus nephritis (LN). In patients with active LN, treatment with intravenous (IV) cyclophosphamide has been shown to reduce the risk of end-stage renal failure with few serious complications compared with high-dose oral prednisone or azathioprine alone [Austin HA III et al. N Engl J Med 1986]. The benefit of long-term treatment with cyclophosphamide has also been shown in more severe patients with diffuse proliferative LN for whom an extended course of pulse cyclophosphamide is more effective than 6 months of pulse methylprednisolone (Figure 1) [Boumpas DT et al. Lancet 1992].
Lupus Nephritis: NIH Study.
IV-CY-L=intravenous cyclophosphamide long course; IV-CY-S=intravenous cyclophosphamide short course; NIH=National Institutes of Health.
Reproduced from The Lancet Vol. 340. Boumpas DT et al. Controlled Trial of Pulse Methylprednisolone Versus Two Regimens of Pulse Cyclophosphamide in Severe Lupus Nephritis, 741–5, Copyright 1992, with permission from Elsevier.
Although cyclophosphamide is effective as induction therapy for LN, maintenance therapy with this agent is inferior to both azathioprine and MMF in terms of event-free (patient and renal) survival, and may result in a higher incidence of hospitalization, amenorrhea, infections, nausea, and vomiting [Contreras G et al. N Engl J Med 2004]. In A Study of Mycophenolate Mofetil (CellCept) in Management of Patients with Lupus Nephritis [ALMS Maintenance], MMF was superior to azathioprine in maintaining a renal response to treatment and in preventing relapse in patients with lupus nephritis who had a response to induction therapy [Dooley MA et al. N Engl J Med 2011]. However, results from other maintenance studies have differed, and, overall, Dr. Balow believes the use of azathioprine is more cost-effective than MMF.
Dr. Balow recommended using cyclophosphamide or MMF as induction therapy for proliferative LN, but cyclophosphamide-based therapy is preferred for severe LN. Treatment of refractory LN should be approached first by swapping cyclophosphamide and MMF; subsequently it may be necessary to consider alternative experimental options [Lehman T et al. ACR 2012 Abstract 621]. For proliferative LN, Dr. Balow recommended maintenance therapy for at least 1 year beyond complete remission, which is defined by quiescent extrarenal disease, stable or improved renal function, stable or improved serologies, remission of proteinuria, and inactive urine sediment.
With the introduction of the revised and validated American College of Rheumatology (ACR) SLE classification criteria, new thought is being directed at SLE treatment goals. Bevra H. Hahn, MD, Univeristy of California, Los Angeles, California, USA, discussed other treatments to induce and maintain improvement and prevent further damage for lupus patients. According to the Systemic Lupus International Collaborating Clinics (SLICC) group, for the classification of SLE, the patient must satisfy at least 4 criteria, including at least 1 clinical criterion and 1 immunologic criterion, or the patient must have biopsy-proven LN in the presence of antinuclear antibodies or anti-ds DNA antibodies [Petri M et al. Arthritis Rheum 2012].
Dr. Hahn characterized musculoskeletal manifestations of lupus as predominantly occurring in the hands and wrists, often presenting with greater pain than the physical exam reveals and with the occurrence of deformities (Jaccoud's arthropahty) in 10% patients. Erosions are generally not present, but if present on routine X-ray or if the patient is positive for anticyclic citrullinated peptide antibodies, clinicians should consider coding the patients as having SLE and RA overlap (rhupus).
Treatment of arthritis should start with antimalarials plus NSAIDS, including topicals—although Dr. Hahn noted that a controlled trial showed little effect compared with placebo [Williams HJ et al. J Rheumatol 1994]. She said if there is no response, add low-dose glucocorticoids or one of the following: methotrexate, azathioprine, leflunomide, MMF, belimumab, abatacept, or tumor necrosis factor inhibitors (tocilizumab).
SLE can lead to a wide range of neurological complications. However, misperceptions about phenomenology and treatment of neurolupus are common. SLICC criteria for diagnosis include seizures, psychosis, mononeuritis, myelitis, peripheral neuropathy, cranial neurophathy, acute confusion state, and possibly depression. A key first step is to determine if the problem is due to lupus or something else; if the cause is lupus, is it vascular or nonvascular, and inflammatory or noninflammatory? European League Against Rheumatism guidelines (1A) state that a magnetic resonance imaging analysis should include T1T2 weighted, contrast, and diffusion-weighted imaging. Glucocorticoid and immunosuppressive therapy are indicated for neuropsychiatric manifestations reflecting an immune/inflammatory process with other causes excluded, including acute confusional state, aseptic meningitis, myelitis, cranial or peripheral neuropathy, psychosis, or optic neuritis. A safe and fast-acting therapy is the best approach.
- © 2012 MD Conference Express®