Summary
Outcomes from the Abatacept Versus Adalimumab Head-to-Head [AMPLE; NCT00929864] trial demonstrated comparable efficacy between subcutaneous abatacept and adalimumab on background methotrexate. This article discusses key efficacy and safety results from the trial.
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
Outcomes from the Abatacept Versus Adalimumab Head-to-Head [AMPLE; NCT00929864] trial demonstrated comparable efficacy between subcutaneous (SC) abatacept and adalimumab on background methotrexate (MTX). Michael E. Weinblatt, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented key efficacy and safety results from the trial.
Dr. Weinblatt said that AMPLE is the first head-to-head study in rheumatoid arthritis (RA) patients that is powered to compare biologic disease-modifying antirheumatic drugs (DMARD) on a background of MTX in subjects who have failed MTX therapy and are naïve to biologic DMARD therapy. The hypothesis was that 12 months of treatment with SC abatacept would be noninferior to adalimumab. The primary endpoint was the proportion of subjects meeting the American College of Rheumatology 20% improvement criteria (ACR20) at 12 months.
The Phase 3b AMPLE study is a randomized, investigator-blinded, 24-month trial with a 12-month primary efficacy endpoint. In total, 646 biologic-naïve patients with active RA and inadequate response to MTX were stratified by disease activity and randomized 1:1 to either abatacept 125 mg SC (without an intravenous load) weekly or adalimumab 40 mg SC biweekly, in combination with a stable dose of MTX.
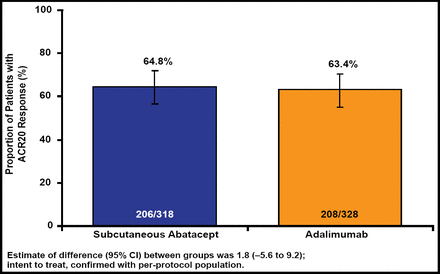
Baseline characteristics were similar in both groups. The 646 patients had a mean disease duration of about 1.8 years. Both abatacept and adalimumab showed comparable efficacy and kinetics of clinical response over the course of 1 year. At 4 weeks, 42.5% of patients in the abatacept group achieved ACR20 response versus 47.6% in the adalimumab group. At 12 months, 64.8% of the abatacept group and 63.4% of the adalimumab group achieved the primary endpoint of ACR20 response, confirming abatacept noninferiority.
Rates for low disease activity (28-joint Disease Activity Score [DAS28]-C-reactive protein [CRP] score ≤3.2) at Year 1 were 59.3% for abatacept and 61.4% for adalimumab. The respective numbers for remission (DAS28-CRP <2.6) were 43.3% versus 41.9%. AMPLE also included measures for changes in radiographic scores and rates of nonprogressors at Year 1. The mean joint space narrowing score (standard deviation [SD]) was 0.28 (1.92) in the abatacept group (n=290) versus 0.39 (2.50) in the adalimumab group (n=289). Numbers for radiographic nonprogressors were 246/290 (84.8%) and 256/289 (88.6%), respectively.
According to Dr. Weinblatt, SC abatacept was noninferior to adalimumab (64.8% vs 63.4%) in the primary outcome measure of ACR20 at 1 year (Figure 1). Comparable responses, including similar onset, were seen across all efficacy variables, including the ACR core components. Other than fewer discontinuations due to adverse events and serious adverse events in the SC abatacept group and significantly (p=0.006) less frequent local injection-site reaction complaints in abatacept patients, safety outcomes were balanced.
SC Abatacept Is Noninferior to Adalimumab.
Reproduced with permission from ME Weinblatt, MD.
- © 2012 MD Conference Express®