Summary
African Americans suffer from lupus-related kidney failure at higher rates compared with individuals without recent African ancestry [Byrne C et al. Am J Kidney Dis 1994; Satko SG et al. Kidney Int Suppl 2005]. This article presents findings from A National Consortium to Explore the Genotypic Basis for End-Stage Renal Disease in Lupus [1RC2-AR058951; P01-AR049084; P01-AI083194].
- Lupus Clinical Trials
African Americans suffer from lupus-related kidney failure at higher rates compared with individuals without recent African ancestry [Byrne C et al. Am J Kidney Dis 1994; Satko SG et al. Kidney Int Suppl 2005]. Robert P. Kimberly, MD, University of Alabama at Birmingham, Birmingham, Alabama, USA, presented findings from A National Consortium to Explore the Genotypic Basis for End-Stage Renal Disease (ESRD) in Lupus [1RC2-AR058951; P01-AR049084; P01-AI083194].
To untangle biological and socioeconomic factors, the researchers took a genetic approach, comparing people of African American ancestry with lupus/ESRD to those with lupus and no nephritis.
The G1 and G2 coding variants in the apolipoprotein L1 gene (APOL1) are strongly and reproducibly associated with focal segmental glomerulosclerosis, HIV-associated collapsing glomerulopathy, and hypertension-attributed ESRD in African Americans [Genovese G et al. Science 2010; Tzur S et al. Hum Genet 2010]. To explore the role of APOL1 in lupus nephritis (LN)-related ESRD, the Consortium tested for associations between APOL1 risk variants and LN-ESRD in a national sample of unrelated African Americans with systemic lupus erythematosus (SLE).
The sample included 668 African American subjects with LN-ESRD (456 with kidney biopsy documentation, 212 physician-reported) and 697 African American subjects with longstanding SLE lacking LN (mean duration of disease was 10.1 years). In cases with LN-ESRD, 87.1% were female, 89% received cytotoxic therapy, mean ± SD age at SLE onset was 26.6±0.4 years and duration of SLE diagnosis to ESRD was 7.2±0.3 years with the median at 5 years. In non-nephritis SLE subjects, 93.5% were female with SLE onset at 35.2±0.8 years of age.
Contrasting all cases with and without ESRD, APOL1 risk variants were significantly associated with LN-ESRD (OR, 2.35; 95% CI, 1.77 to 3.3; p=4.25E-9); significant differences in association were not observed when comparing cases with or without kidney biopsy documentation to SLE subjects without LN. The duration of SLE onset to ESRD for those with the G1/G2 variants was 5.49±0.54 years (median=4). For those without the variants, it was 7.78±0.37 years (median=6; p<0.05).
The study demonstrates strong association between both APOL1 G1 and G2 variants and LN-associated ESRD in African Americans. It appears likely that APOL1 G1 and G2 coding variants, which are rare in European populations, contribute to nephropathy progression in LN-ESRD, as well as other nondiabetic etiologies of ESRD. These variants and their higher prevalence in those with African ancestry may explain, in part (Figure 1), a higher prevalence of severe LN in African Americans.
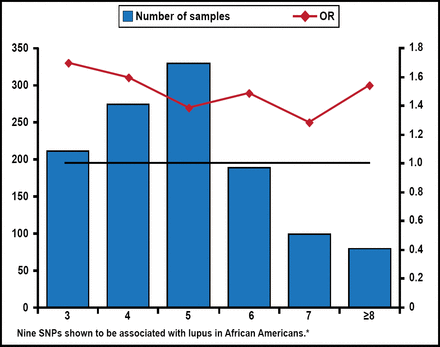
The Genetic Load Associated with Lupus in African Americans.
*Source: Vaughn SE et al. J Leukoc Biol 2012. SNP=single-nucleotide polymorphism.
Reproduced with permission from RP Kimberly, MD.
- © 2012 MD Conference Express®