Summary
In a platelet function substudy of A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects [TRILOGY ACS; NCT00699998] trial, patients with an acute coronary syndrome treated with prasugrel experienced significantly lower platelet reactivity compared with patients treated with clopidogrel. However, there was no relationship between platelet reactivity and the occurrence of ischemic outcomes, nor were there significant differences in the rates of the composite endpoint of cardiovascular death, myocardial infarction and stroke, or TIMI major bleeding between the 2 treatment groups enrolled in the platelet function substudy.
- Coronary Artery Disease
- Cardiology Clinical Trials
In a platelet function substudy of A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects [TRILOGY ACS; NCT00699998] trial, patients with an acute coronary syndrome (ACS) treated with prasugrel experienced significantly lower platelet reactivity compared with patients treated with clopidogrel. However, there was no relationship between platelet reactivity and the occurrence of ischemic outcomes, nor were there significant differences in the rates of the composite endpoint of cardiovascular death, myocardial infarction (MI) and stroke, or TIMI major bleeding between the 2 treatment groups enrolled in the platelet function substudy. Paul Gurbel, MD, Sinai Hospital of Baltimore, Baltimore, Maryland, USA, presented the results of this novel mechanistic evaluation of platelet function in the TRILOGY ACS trial.
The substudy included 2564 patients enrolled in the TRILOGY ACS trial that randomized patients with unstable angina (UA) or non-ST segment elevation myocardial infarction (NSTEMI) initially managed medically without revascularization in the TRILOGY ACS trial to prasugrel or clopidogrel. Prasugrel was administered with dose adjustment based on weight or age: patients aged <75 years and weighing ≥60 kg received 10 mg/day, while those aged ≥75 years (regardless of weight) and those aged <75 years who weighed <60 kg received 5 mg/day. All patients in the clopidogrel group received 75 mg/day.
In the substudy group, platelet function was measured using the VerifyNow P2Y12 assay at baseline, and at 2 hours, and 1, 3, 6, 12, 18, 24, and 30 months after randomization. The primary efficacy endpoint was a composite of cardiovascular death, MI, and stroke through 30 months. Key secondary endpoints included all-cause death and MI. Approximately 20% of patients were aged ≥75 years, 39% were female, 33% had UA, and 67% had NSTEMI.
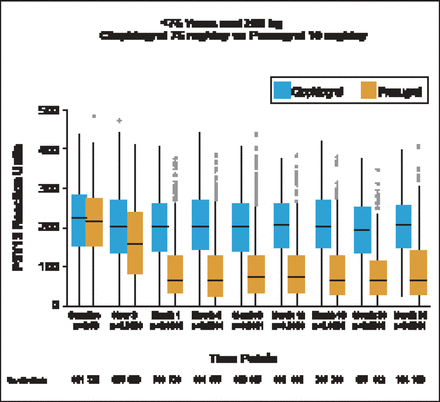
Patients treated with prasugrel (10 mg/day) had significantly (p<0.0001) lower P2Y12 reaction units (PRU) compared with those treated with clopidogrel, starting at 2 hours and persisting throughout the 30 month study (Figure 1). The median PRU values at 30 days were 64 (IQR 33 to 128) in the prasugrel group versus 200 (IQR, 141 to 260) in the clopidogrel group. Patients aged <75 years who weighed <60 kg and received prasugrel 5 mg/day also had significantly (p<0.001) lower PRUs compared with patients receiving clopidogrel beginning at Month 1. Compared with patients receiving 75 mg/day clopidogrel, PRU was significantly lower in patients aged ≥75 years receiving 5 mg prasugrel beginning at Month 1 (p<0.001) and continuing to Month 24 (p=0.02). Median 30-day PRU values were significantly lower in patients treated with 10 mg prasugrel versus those treated with 5 mg prasugrel (p<0.001).
Median On-Treatment PRU Through 30 Months.
Reproduced with permission from PA Gurbel, MD.
The percentage of patients with high platelet reactivity (HPR), defined as >208 PRU (a cutpoint that has been shown to identify patients at higher risk of future ischemic events) was greater in clopidogrel-treated patients. In unadjusted analyses, the primary composite endpoint of cardiovascular death, MI, and stroke through 30 months occurred in significantly more patients with HPR compared with patients without HPR by Kaplan-Meier estimates defined at cutpoints of >178 PRU, and >208 PRU, and evaluating PRU as a time-dependent covariate. However, when adjusting for differences there was no difference in outcomes between patients with and without HPR using any of these definitions (Table 1). Numerically higher outcomes were noted for MI events and all-cause death in patients with HPR compared with patients without HPR but differences were not statistically signifiacant. In a multivariate analyses, platelelet reactivity was not independently associated with ischemic event occurrence.
Relationship of PRU and Ischemic Outcomes.
Dr. Gurbel noted that PRU measurements were not always performed in close proximity to clinical event occurrence. Although prasugrel was associated with lower PRU values irrespective of age, weight, and dose relative to clopidogrel, ischemic event occurrence did not differ between treatment groups. He said that, in this group of medically managed ACS patients, it does not appear that the ADP-P2Y12 receptor interaction played as significant of a role in ischemic event occurrence as in the population of patients treated with stents.
- © 2012 MD Conference Express®