Summary
Drug-coated balloons (DCB) offer homogeneous drug distribution to the vessel wall without the need for a permanent implant. This article discusses issues related to DCB development and evidence.
- Thrombotic Disorders
- Interventional Techniques & Devices
Drug-coated balloons (DCB) offer homogeneous drug distribution to the vessel wall without the need for a permanent implant. Bruno Scheller, MD, Universitätsklinikum des Saarlandes, Homburg/Saar, Germany, discussed issues related to DCB development and evidence.
Endovascular Indications
In the Local Taxane with Short Time Contact for Reduction of Restenosis in Distal Arteries [THUNDER; Tepe G et al. N Engl J Med 2008] trial, patients with femoropopliteal artery stenosis or occlusion were randomly assigned to treatment with paclitaxel-coated balloons (n=48), uncoated balloons with paclitaxel in the contrast medium (n=52), or uncoated balloons without paclitaxel (control; n=54). Mean late lumen loss at 6 months was significantly lower in the paclitaxel-coated balloon group (0.4 mm) versus the control group (1.7 mm; p<0.001).
In five trials of DCB versus percutaneous transluminal angioplasty (PTA) in the superficial femoral artery (SFA), patients treated with DCB had significantly reduced restenosis at 6 and 12 months and clinical and functional benefit maintained up to 2 or more years [BIOLUX P-I, NCT01221610; FEMPac, NCT00472472; LEVANT I, NCT00930813; PACIFIER, NCT01083030; THUNDER, NCT00156624]. A subanalysis of the Paclitaxel-coated Balloons in Femoral Indication to Defeat Restenosis [PACIFIER] trial showed that DCB benefited patients with de novo stenosis and total occlusion in the SFA, independent of lesion length [Werk M et al. Circ Cardiovasc Int 2012. In press].
A nonrandomized study of patients with below-the-knee (BTK) lesions ∼17 to 18 cm long reported a restenosis rate after 3 months of 27.4% with DCB versus 69% with PTA [Schmidt A et al. Cath Card Int 2010; Schmidt A et al. J Am Coll Cardiol 2011]. The randomized Drug Eluting Balloon in Peripheral Intervention for Below the Knee Angioplasty Evaluation [DEBATE-BTK; NCT01558505] trial demonstrated significantly reduced 12-month restenosis and reocclusion with DCB versus conventional balloon angioplasty in patients with diabetes and critical limb ischemia [Liistro F et al. LINC 2012].
In a nonrandomized series of patients with intracranial in-stent restenosis (ISR), high-grade restenosis rate occurred in 9% with DCB versus 50% with conventional balloon treatment [Vajda Z et al. Am J Neuroradiol 2011]. In patients with arteriovenous fistulas, target lesion primary patency was 70% with DCB versus 25% with conventional balloon at 6 months (p<0.001) [Karnabatidis D. CIRSE 2011 Abstract 1905.3].
Coronary Indications
Compared with implantation of another DES, DCBs for coronary ISR may eliminate the need for a second or third stent, and reduce the need for prolonged dual antiplatelet therapy. Scheller et al. [N Engl J Med 2006] found that in-segment late lumen loss in DES patients treated for ISR was 0.03 mm with DCB versus 0.74 mm with a conventional balloon. Follow-up to 6 years demonstrated reduced target lesion revascularization with DCB (9.3%) versus conventional balloon (38.9%; p=0.004) [Scheller B et al. JACC Cardiovasc Interv 2012]. In patients with coronary ISR from a bare-metal stent (BMS), Unverdorben et al. [Circulation 2009] reported reduced in-stent late loss (0.19 mm vs 0.45 mm; p=0.01), in-segment late loss (0.17 mm vs 0.38 mm; p=0.03), and in-segment restenosis (7.0% vs 20.3%; p=0.06) with DCB versus DES.
Patients with sirolimus-eluting ISR had significantly reduced restenosis (8.7% vs 62.5%; p=0.0001) with DCB versus a conventional balloon [Habara S et al. JACC Cardiovasc Interv 2011]. The Paclitaxel-Eluting PTCA-Balloon Catheter to Treat Small Vessel Coronary Artery Disease [PEPCAD I; Unverdorben M et al. Clin Res Cardiol 2010] study in patients with de novo coronary lesions reported 6-month restenosis of ∼5.5% with DCB and ∼45% with DCB plus bare-metal stent (p=0.0001 for both).
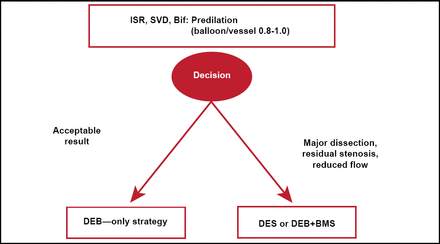
The German consensus group recommendations for DCB use are shown in Figure 1 [Kleber FX et al. EuroIntervention 2011].
German Consensus Group Recommendations for DCB Use.
Source: Kleber FX et al. EuroIntervention 2011.
DCBs can be used for a variety of endovascular and coronary indications. Results thus far have been promising for both endovascular therapy and treatment of ISR in the coronary bed. De novo disease is more of a challenge but trials are ongoing to clarify DCB use for this indication. Prof. Scheller said that DCBs are not a replacement for DES but may become an important new option in endovascular and coronary intervention. Compared with bioabsorbable stents, DCB have much better evidence from randomized clinical trials and large registries. However, both methods appear complementary toward the goal of avoiding permanent implants. According to Prof. Scheller, DCB and bioabsorbable stents represent technology for a new age of vascular therapy with the aim of leaving nothing behind.
- © 2012 MD Conference Express®