Summary
Approximately 30% to 40% of ischemic strokes are classified as cryptogenic because a recognized cause is not identified [Sacco RL et al. Ann Neurol 1989]. Paradoxical embolism due to patent foramen ovale (PFO) is a possible cause of ischemic stroke, particularly in young cryptogenic stroke patients. Several presentations at TCT 2012 added important data to the growing literature about PFO closure and highlighted how elusive secondary stroke prevention with device therapy remains.
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cardiology Clinical Trials
Approximately 30% to 40% of ischemic strokes are classified as cryptogenic because a recognized cause is not identified [Sacco RL et al. Ann Neurol 1989]. Paradoxical embolism due to patent foramen ovale (PFO) is a possible cause of ischemic stroke, particularly in young cryptogenic stroke patients. However, it is often difficult to establish a firm etiological association [Horner S et al. J Neurol 2012] and optimal treatment for secondary prevention in patients with cryptogenic stroke and PFO is still undefined [O'Gara PT et al. J Am Coll Cardiol 2012]. Several presentations at TCT 2012 added important data to the growing literature about PFO closure and highlighted how elusive secondary stroke prevention with device therapy remains.
The PC-Trial: Patent Foramen Ovale Closure Versus Medical Therapy
The Patent Foramen Ovale and Cryptogenic Embolism trial [PC-Trial; NCT00166257] presented by Stephan Windecker, MD, Swiss Cardiovascular Center, Bern, Switzerland, tested whether percutaneous closure of PFO using the Amplatzer PFO Occluder would be superior to medical treatment for secondary prevention of thromboembolism in patients with cryptogenic stroke and peripheral embolism.
The trial randomized 414 patients to PFO closure (n=204) with the Amplatzer device along with acetylsalicylic acid and ticlopidine or clopidogrel for 6 months or to optimal medical treatment (n=210) with oral anticoagulation or antiplatelet therapy. Patients had to be <60 years of age and have clinically and neuroradiologically verified ischemic stroke or transient ischemic attack (TIA) with a documented corresponding intracranial ischemic lesion or extracranial peripheral thromboembolism. Patients with any other cause for a thromboembolic event were excluded.
The primary endpoint was the composite of death from any cause, nonfatal stroke, TIA, and peripheral embolism. The secondary endpoints were myocardial infarction (MI), new atrial fibrillation, rehospitalization for PFO, and device-related problems.
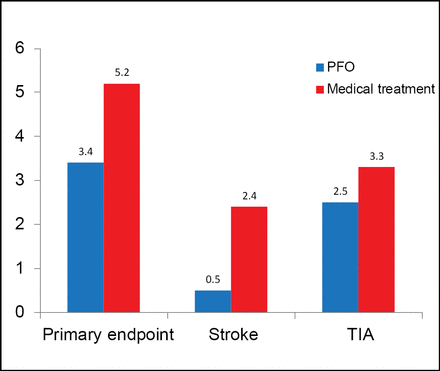
PFO closure was successful in 96.9% of patients; 95.9% had effective closure at 6 months. Residual shunt was absent in 91.7%, minimal in 6.2%, moderate in 0.7%, and severe in 1.4% of PFO closure patients. At a mean follow-up of 4 years, the primary endpoint occurred in 3.4% of patients (142) in the PFO closure arm versus 5.2% (131) in the medical treatment arm, a nonsignificant 37% relative risk reduction (RRR) with PFO closure (HR, 0.63; 95% CI, 0.24 to 1.62; p=0.34; Figure 1).
Results.
RRR=relative risk reduction; TIA=transient ischemic attack.
Stroke occurred less frequently in the PFO closure group than in the medical treatment group, but the difference was not statistically significant (0.5% vs 2.4%; HR, 0.20; 95% CI, 0.02 to 1.72; p=0.14). Similar findings were noted for TIA (HR, 0.71; 95% CI, 0.23 to 2.24; p=0.56). There were no significant differences between PFO closure and medical treatment in MI (1.0% vs 0.5%; HR, 2.04; 95% CI, 0.19 to 22.5; p=0.56) and PFO-related hospitalizations (6.4% vs 6.2%; HR, 1.02; 95% CI, 0.48 to 2.21; p=0.95).
There were no significant differences between PFO closure and medical treatment in the rate of bleeding complications (3.4% vs 5.7%; p=0.25). Atrial fibrillation occurred in 2.5% of PFO closure patients versus 1.0% of medically treated patients (p=0.25). There were no significant differences in thromboembolic events between the groups.
Study limitations included a lower than expected event rate in the medical therapy arm (5.2% vs 12%), inadequate power with <40% power to detect a hypothesized 66% RRR, long recruitment duration, and high attrition rate.
RESPECT: Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment
John D. Carroll, MD, University of Colorado, Denver, Colorado, USA, presented findings from The Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment trial [RESPECT; NCT00465270].
Like the PC-Trial, the multicenter, prospective, randomized RESPECT trial evaluated the potential benefit of the Amplatzer™ PFO Occluder compared with medical therapy for the prevention of recurrent embolic stroke in patients with a prior cryptogenic stroke within 270 days and a documented PFO.
The primary endpoints were recurrence of a nonfatal ischemic stroke, fatal ischemic stroke, or all-cause mortality within 45 days. The trial was event-driven and enrollment was stopped once 25 primary endpoint events had occurred.
A total of 980 patients aged 18 to 60 years were enrolled between 2003 and 2011, with 499 randomized to the device group and 481 to the medical group. Baseline demographic and medical characteristics were similar between the 2 groups. Total exposure in patient-years was 1375 years of follow-up in the device group (with 48 dropouts) and 1184 in the medical group (with 90 dropouts).
The primary analysis using the raw count of the intention-to-treat (ITT) cohort was deemed invalid because of less exposure due to the greater dropout rate in the medical group. The protocol specified that in the event of unequal dropout, the survival functions for the time-to-endpoint event for each treatment group would be used to provide an exposure stratified comparison. In the ITT analysis, there was a nonsignificant 50.8% RRR in stroke with device closure versus medical therapy (HR, 0.492; 95% CI, 0.217 to 1.134; log-rank p=0.0825).
Exploratory analyses of supplementary per protocol and as-treated patients (based on 21 stroke events) showed statistical significance. In the per protocol cohort, the risk reduction of stroke in favor of the device was 63.4% (HR, 0.366; 95% CI, 0.141 to 0.955; log-rank p=0.0321) and in the as-treated cohort there was a significant 72.7% risk reduction of stroke in favor of device closure (HR, 0.273; 95% CI, 0.100 to 0.747; log-rank p=0.0067).
Dr. Carroll concluded that results of the RESPECT trial have substantial import for the treatment of carefully selected patients with a history of cryptogenic stroke and PFO.
Placing the RESPECT and PC Trials in Context
The PC and the RESPECT trials found no significant difference in their primary endpoints. Results from both were affected by several limitations, including low event rate, exclusion of highest-risk patients, available off-label closure, differential dropout rate, and subgroup analysis with only 25 events (RESPECT).
Jonathan M. Tobis, University of California, Los Angeles, California, USA, offered a review of the current state of the science for secondary stroke prevention with device closure. He noted that a PFO can be found in 20% to 25% of the population and some, but not all, case-control studies have found an increased incidence of PFO in patients with cryptogenic stroke (Table 1) [Irwin B, Ray S. Cardiovasc Ther 2012].
Association of PFO and Cryptogenic Stroke in Young Adults (<55 years of age).
His remarks focused on three major randomized, open-label PFO trials: Evaluation of the STARFlex® Septal Closure System in Patients with a Stroke or TIA Due to the Possible Passage of a Clot of Unknown Origin Through a Patent Foramen Ovale (PFO) [CLOSURE I], Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], and GORE HELEX™ Septal Occluder for Patent Foramen Ovale (PFO) Closure in Stroke Patients [REDUCE; NCT00738894].
The CLOSURE I trial compared closure with the STARFlex Septal Closure System versus medical management, the RESPECT trial evaluated percutaneous PFO closure, using the Amplatzer™ PFO Occluder, against medical management and the REDUCE trial is investigating whether PFO closure with the GORE HELEX Septal Occluder plus antiplatelet medical management is safe and effective at reducing the risk of recurrent stroke or image-confirmed TIA.
The CLOSURE I trial randomized 909 patients; RESPECT 980; and REDUCE, 664 (estimated enrollment) randomized to aspirin, or aspirin plus dipyridamole or clopidogrel. According to Dr. Tobis, PFO stroke trials share the challenges of low event rates (<2% recurrent stroke/year), availability of off-label PFO closure, and exclusion of highest-risk patients due to nonrandomization. The RESPECT trial closed in January 2012 with a total of 25 events in 9 years [Carroll JD et al. TCT 2012]. The CLOSURE I trial showed that patients with cryptogenic stroke or TIA who had a PFO did not receive a greater benefit from closure with a device than from medical therapy alone [Furlan AJ et al. N Engl J Med. 2012]. Results are shown in Table 2.
CLOSURE I: Kaplan-Meier Event Rates for Primary Endpoint at 2 Years*.
Science Advisors' Statement
Stroke is a compelling public health problem and a major contributor to morbidity and mortality worldwide. There is ample observational data suggesting an increased incidence of PFO in patients with cryptogenic stroke. Furthermore, there has been rapid development of closure devices as well as the technical capacity to safely deploy these devices in the hope of preventing recurrent strokes. However, despite observational data that appears to favor mechanical closure over medical therapy [Kisios GD et al. Stroke 2012], randomized clinical trial data has been disappointing. In the CLOSURE I trial, there was no significant benefit with device closure as compared with medical therapy for prevention of recurrent stroke or TIA among patients with cryptogenic stroke or TIA with PFO [Furlan AJ et al. N Engl J Med 2012]. Similarly, the PC-Trial and RESPECT trial failed to meet their primary endpoints. Large relative risk reductions in stoke may be apparent and seemingly impressive due to the small number of overall events, yet statistical significance in the randomized trials has not been realized.
Closure of PFOs to reduce the risk of thromboembolism remains both attractive and elusive. Many providers logically believe that PFO closure should prevent strokes and thereby have lost equipoise. However, the many instances where clinical trials have shattered the prevailing wisdom or treatment paradigms should not be forgotten—suppression of ventricular arrhythmias, vitamin E replacement, distal embolic protection in acute myocardial infarction intervention, and routine percutaneous intervention in patients with stable coronary artery disease, to name a few. This notwithstanding, the difficulties in conducting PFO closure clinical trials must be recognized, such as low event rates, variable optimal medical treatment, and patient selection, that make conventional research paradigms and alpha-levels (p<0.05) difficult to apply. Clearly, the trial populations to date have not been able to selectively define a subpopulation of cryptogentic stoke patients with a PFO who clearly benefit from closure and the group of patients with PFO as a whole has not shown benefit. In today's tough economic times, the reimbursement for PFO closure is unlikely to find a place based on the currently available data. As in the past, ongoing studies must be completed to further address this critical question and, until then, the body of evidence currently available must be applied, one patient at a time.
- © 2012 MD Conference Express®