Summary
The original Drug-eluting Stents for Unprotected Left Main Stem Disease [ISAR-Left Main] study found no significant difference in outcomes for patients with unprotected left main coronary artery stenosis (uLMCS) who were treated with first generation paclitaxel-eluting versus sirolimus-eluting stents [Mehilli J et al. J Am Coll Cardiol 2009]. The objective of the current ISAR-LEFT MAIN 2 trial [NCT00598637] was to compare the performance of zotarolimus-eluting stent versus everolimus-eluting stent in patients with uLMCS lesions, using a noninferiority design.
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology Clinical Trials
The original Drug-eluting Stents for Unprotected Left Main Stem Disease [ISAR-Left Main] study found no significant difference in outcomes for patients with unprotected left main coronary artery stenosis (uLMCS) who were treated with first generation paclitaxel-eluting versus sirolimus-eluting stents [Mehilli J et al. J Am Coll Cardiol 2009]. Since then, the American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions updated the guidelines for percutaneous coronary intervention (PCI) of uLMS to include a class IIa or IIb indication for those patients with uLMCS lesions who have nonextensive coronary disease and are at a low stenting risk or a high surgical risk [Levine GN et al. Circulation 2011]. This inclusion into the PCI practice guidelines has led to more widespread use of PCI for the treatment of uLMS.
The second generation zotarolimus-eluting stent (ZES) and everolimus-eluting stent (EES) have been shown to perform better than first-generation drug-eluting stents (DES) in nearly all coronary lesion subsets, however there has been no direct comparison of these two platforms in uLMS [Stone GW et al. New Engl J Med 2010; von Birgelen C et al. J Am Coll Cardiol 2012; Serruys PW et al. N Engl J Med 2010; Kim YH et al. JACC Cardiovasc Interv 2012].
The objective of the current ISAR-LEFT MAIN 2 trial [NCT00598637] presented by Julinda Mehilli, MD, Klinikum der Universität, Munich, Germany, was to compare the performance of ZES versus EES in patients with uLMCS lesions, using a noninferiority design.
The trial randomized 650 patients with uLMCS to PCI using ZES (n=324) or EES (n=326) after pretreatment with 600 mg of clopidogrel. Follow-up assessments included angiography at 8 months in 237 (73%) patients in the ZES group and 226 (69%) patients in the EES group, and clinical evaluation at 12 months in all patients in both groups. The primary endpoint was the incidence of major adverse cardiac events (MACE), defined as the composite of death, myocardial infarction, and target lesion revascularization at 1-year follow-up. The secondary endpoints were the incidence of definite or probable stent thrombosis at 1 year and angiographic restenosis at 6 to 9 months. The noninferiority margin was calculated at 9%.
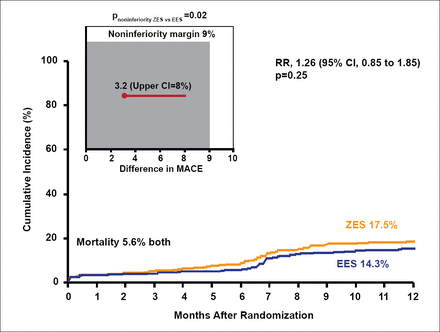
At 1-year follow-up, MACE occurred in 17.5% of the ZES group and 14.3% of the EES group (RR, 1.26; 95% CI, 0.85 to 1.85; p=0.25; Figure 1). The mortality rate was 5.6% in both groups. The ZES met the prespecifed noninferiority margin (noninferiority p=0.02).
Major Adverse Cardiac Events.
Reproduced with permission from J Mehilli, MD.
Definite and probable stent thrombosis occurred in 0.6% and 0.3% of ZES patients, respectively, and in 0.6% and 0.0% of EES patients, respectively. There was no significant difference between ZES-treated and EES-treated patients with regard to angiographic restenosis (21.5% vs 16.8%; p=0.2) or clinical restenosis (11.7% vs 9.4%; p=0.35) respectively.
The ISAR-LEFT MAIN 2 trial results show that the use of second-generation DES in unprotected left main coronary artery lesions in relatively unselected patients is feasible, safe, and effective. Both stents, the ZES and the EES, provided similar clinical and angiographic outcomes at 1-year follow-up in this high-risk patient population.
- © 2012 MD Conference Express®