Summary
Angioplasty with a paclitaxel-eluting balloon was as effective as implantation of another drug-eluting stent for patients who have in-stent restenosis in the presence of a “limus”-eluting stent. Both procedures were significantly better than plain old balloon angioplasty, according to the results of the Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis: 3 Treatment Approaches [ISAR-DESIRE 3; NCT00987324] trial.
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology Clinical Trials
- Interventional Radiology
Angioplasty with a paclitaxel-eluting balloon (PEB) was as effective as implantation of another drug-eluting stent (DES) for patients who have in-stent restenosis (ISR) in the presence of a “limus”-eluting stent. Both procedures were significantly better than plain old balloon angioplasty (POBA), according to the results of the Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis: 3 Treatment Approaches [ISAR-DESIRE 3; NCT00987324] trial.
DES have been used for more than a decade, but the optimal treatment for ISR is unknown, said Robert A. Byrne, MBBCh, PhD, Deutsches Herzzentrum, Technische Universität, Munich, Germany, who reported on the study. He said that drug-eluting balloon (DEB) angioplasty has the advantage of avoiding additional stent layers, and small studies have shown promise for the treatment in patients who have ISR with a bare-metal stent. However, the role of this therapy for ISR in the presence of a DES is poorly defined. ISAR-DESIRE 3 was designed to compare the antirestenotic efficacy of 3 treatments of limus-eluting ISR: angioplasty with a PEB, implantation of a paclitaxel-eluting stent (PES), and traditional balloon angioplasty.
The trial enrolled 402 patients at 3 centers in Germany. All patients had ISR of more than 50% in a limus-eluting stent in the presence of symptoms/signs of ischemia. Patients with left main stem disease, acute ST-elevation myocardial infarction (MI), or cardiogenic shock were excluded. The patterns of restenosis at baseline were well balanced across the groups, with a focal pattern in two-thirds of patients and a nonfocal pattern in one-third.
The patients were randomly assigned in a 1:1:1 manner to the 3 treatment groups. The primary endpoint was percentage diameter restenosis on follow-up angiography at 6 to 8 months. Secondary efficacy endpoints were binary restenosis and target lesion revascularization (TLR). Safety endpoints were target lesion thrombosis and a composite of death and MI.
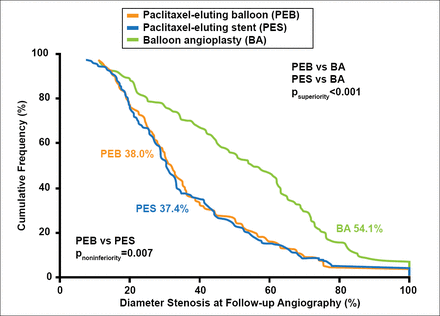
On follow-up angiography, the percentage restenosis was noninferior between the PEB and PES groups (38.0% vs 37.4%, respectively (p for noninferiority=0.007; Figure 1). Both the PEB and PES groups had significantly lower percentage of restenosis when compared with the POBA group (54.1%; p<0.001). The results for the secondary efficacy endpoints followed a similar pattern. Binary restenosis (percentage of patients with restenosis >50%) was found in 26.5% of the patients in the PEB group and 24.0% of the patients in the PES group (p=0.61) compared with 56.7% of the patients in the POBA group (p<0.001). The rates (TLR) were 22.1% for the PEB group, 13.5% for the PES group (p=0.09), and 43.5% for the POBA group (p<0.001).
Primary Endpoint.
Reproduced with permission from RA Byrne, MBBCh, PhD.
In the safety analysis, the rates of death/MI and target lesion thrombosis were low and similar among all 3 groups.
Prof. Byrne noted that the results of the study are limited to limus-eluting stent ISR and cannot be extrapolated to PES ISR. However, he added that there is no compelling reason to believe that the findings would differ substantially.
The researchers concluded that because DEB angioplasty obviates the need for additional stent implantation, this new treatment should be the default strategy for patients who have limus-eluting stent ISR. The European guidelines currently recommend DEB therapy for use only with bare-metal stents.
- © 2012 MD Conference Express®