Summary
This article reviews appropriate clinical indications for transcatheter aortic valve replacement (TAVR) and ongoing controversies regarding the procedure. An appropriate clinical indication for TAVR is severe symptomatic aortic stenosis and either surgical contraindication with a probable life expectancy of >1 year and no contraindications for TAVR due to frailty or comorbidities. Patients at high risk for both surgical aortic valve replacement and TAVR may also be a candidate for TAVR if the procedure is approved by a multidisciplinary team and felt to be the best option.
- Interventional Techniques & Devices
- Valvular Disease
Martyn R. Thomas, MD, St Thomas Hospital, London, United Kingdom, reviewed appropriate clinical indications for transcatheter aortic valve replacement (TAVR) and ongoing controversies regarding the procedure. According to Dr. Thomas, an appropriate clinical indication for TAVR is severe symptomatic aortic stenosis (AS) and either surgical contraindication with a probable life expectancy of >1 year and no contraindications for TAVR due to frailty or comorbidities. Patients at high risk for both surgical aortic valve replacement (SAVR) and TAVR may also be a candidate for TAVR if the procedure is approved by a multidisciplinary team and felt to be the best option.
PARTNER Cohort B: TAVR Versus Standard Therapy
Dr. Thomas said that TAVR should be performed in inoperable patients to prolong survival and improve quality of life (QoL) if the procedure is appropriately cost effective for the particular healthcare system and country. In the Placement of Aortic Transcatheter Valve Trial [PARTNER; NCT00530894], as compared with standard therapy, TAVR led to a significant reduction in all-cause mortality at 2 years (43.3% vs 68.0%; HR, 0.56; 95% CI, 0.43 to 0.73; p<0.001) [Makkar RR et al. N Engl J Med 2012]. TAVR also significantly improved QoL over 12 months (p<0.001) [Reynolds MR et al. Circulation 2011]. Dr. Thomas calculated quality adjusted life years (QALYs) after TAVR at 1.4 compared with 0.4 QALYs with medical therapy alone. The incremental cost-effectiveness ratio for TAVR based on the cost per QALY gained over standard therapy was US $61,889 [Reynolds MT et al. Circulation 2012]. As such, Dr. Thomas proposed that TAVR be considered the standard of care for patients with severe symptomatic AS with a probable life expectancy of >1 year who are ineligible for SAVR.
PARTNER Cohort A: TAVR in High-Risk Surgical Patients
Dr. Thomas said that TAVR might be appropriate in patients at high risk for SAVR because it is less invasive, offers similar survival and QoL benefits to SAVR, and has also been shown to be cost effective. In PARTNER Cohort A, all-cause mortality at 2 years was similar with TAVR (33.9%) and SAVR (35.0%; p=0.78) [Kodali SK et al. N Engl J Med 2012].
The Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score improved more rapidly with TAVR, but was similar for the 2 groups at 6 and 12 months. Patients treated with transfemoral TAVR had a significantly greater improvement in health status at 1 month versus SAVR (difference, 9.9 points; 95% CI, 4.9% to 14.9%; p<0.001), with no significant difference at 6 and 12 months [Reynolds MR et al. J Am Coll Cardiol 2012]. Length of hospital stay in mean days was significantly longer with SAVR versus transfemoral TAVR (16.4 days vs 10.2 days, respectively; p<0.001). Calculation of the cost per QALY gained suggested that TAVR was less expensive and more effective than SAVR. The same was not true of the transapical approach, which resulted in worse QoL and was more expensive than SAVR. Data from Dr. Thomas and his colleagues showed poorer initial QoL with transapical versus transfemoral TAVR, but by 1 year QoL was similar regardless of access.
Ongoing Controversies
Appropriate selection of patients for TAVR in Europe remains a source of some controversy given concerns that TAVR is applied more widely than evidence would support. The 2012 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) Guidelines on the management of valvular heart disease do not mention the European System for Cardiac Operative Risk Evaluation (EuroSCORE) or STS score when discussing TAVR indications [Vahanian A et al. Eur Heart J 2012; Eur J Cardiothorac Surg 2012]. The ESC/EACTS Guidelines recommend the following: TAVR should be undertaken only by a multidisciplinary heart team; TAVR is indicated in patients with severe symptomatic AS who are not suitable for SAVR as assessed by a heart team and who are likely to gain improved QoL and have a life expectancy of >1 year; TAVR should be considered in high-risk patients with severe symptomatic AS for whom TAVR is favored by the heart team.
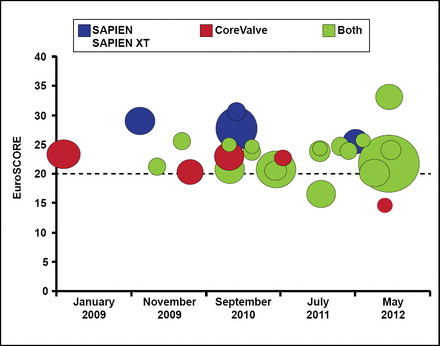
An analysis of the EuroSCORE over the last 5 years suggests that there has not been a significant change in the risk profile of patients undergoing TAVR, according to Dr. Thomas (Figure 1). He noted that while there were patients with EuroSCORE <20 in the SOURCE XT registry who underwent TAVR, several important comorbidities are not considered in the EuroScore assessment and 72.2% of patients had at least 1 of these [Thomas M et al. Circulation 2011].
Analysis of EuroScore in Publications with >200 Patients.
Reproduced with permission from MR Thomas, MD.
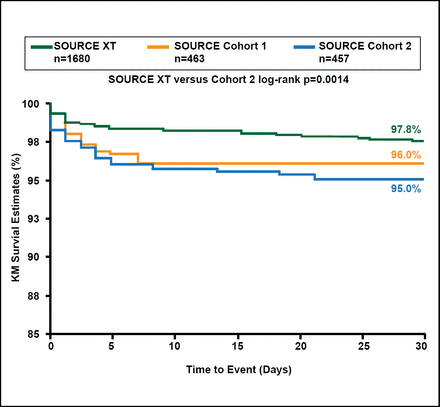
Stroke appeared to be an issue in the PARTNER TAVR and SAVR cohorts. The frequency of overall neurologic events (strokes and transient ischemic attacks) at 2 years was significantly higher with TAVR than with SAVR (11.2% vs 6.5%; p=0.05), although there was no significant difference in overall stroke with TAVR versus SAVR (HR, 1.22; 95% CI, 0.67 to 2.23; p=0.52) [Kodali SK et al. N Engl J Med 2012]. The rate of stroke was higher after TAVR compared with SAVR (13.8% vs 5.5%, p=0.01) [Makkar RR et al. N Engl J Med 2012]. Patients with stroke in the TAVR cohort had improved QoL, but those in the SAVR cohort did not. Stroke rates were lower in the SOURCE XT Registry (Sapien XT) versus the SOURCE Registry (Sapien; Figure 2).
Stroke at 30 Days with the Transfemoral Approach.
Reproduced with permission from MR Thomas, MD.
Continued innovation is resulting in improved replacement valves. The Sapien 3, a balloon-expandable valve scheduled to enter clinical trials by the end of 2012, is expected to improve ease of use and procedure-related adverse events. Similarly, there is increasing experience with valve-in-valve treatment for failing aortic, mitral, or tricuspid bioprostheses.
Another ongoing controversy is the name of the procedure: transcatheter aortic valve replacement (TAVR) versus transcatheter aortic valve implantation (TAVI). Dr. Thomas believes there should be no debate about this issue—the name of a medical technique should be based on an accurate description of the procedure and not on issues related to reimbursement. Therefore, he says the appropriate name is TAVI.
Dr. Thomas concluded that TAVR should be considered the standard of care for the inoperable patient in the absence of futility and considered in high-risk surgical patients if deemed appropriate by a multidisciplinary team. TAVR should only be performed for these indications if the procedure is affordable in the individual healthcare system. There is some evidence of a changing patient population in Europe but little evidence of major risk creep. A more accurate measure of risk in high-risk patients with AS is required. Stroke and paravalvular leak remain important issues that improvements in technology and procedural technique may help reduce.
- © 2012 MD Conference Express®