Summary
Fully bioresorbable scaffolds (BRS) offer a revolutionary treatment approach within the field of interventional cardiology. Since BRS devices provide temporary mechanical support to the vessel wall and then subsequently disappear, the technology has the potential to overcome many of the safety concerns associated with metallic drug-eluting stents, such as late stent thrombosis, prevention of late lumen vessel enlargement, and difficulties with surgical revascularization and imaging artifact with multislice computed tomography [Bourantas CV et al. Int J Cardiol 2012; Serruys PW et al. Lancet 2009]. This article discusses the advances in BRS over the last 5 to 6 years.
- Lipid Disorders
- Interventional Techniques & Devices
Fully bioresorbable scaffolds (BRS) offer a revolutionary treatment approach within the field of interventional cardiology. Since BRS devices provide temporary mechanical support to the vessel wall and then subsequently disappear, the technology has the potential to overcome many of the safety concerns associated with metallic drug-eluting stents (DES), such as late stent thrombosis, prevention of late lumen vessel enlargement, and difficulties with surgical revascularization and imaging artifact with multislice computed tomography [Bourantas CV et al. Int J Cardiol 2012; Serruys PW et al. Lancet 2009]. They may possibly deliver even more clinical benefits [Onuma Y et al. Circ J 2011]. Patrick W. Serruys, MD, PhD, Erasmus University, Netherlands, discussed the advances in BRS over the last 5 to 6 years.
Bourantas et al. [Curr Cardiol Rep 2012] noted that the potential advantages of this rapidly developing technology have led to a drive by industry to develop several types of BRS. Hence, numerous scaffolds are available today with different compositions (eg, metallic alloy or polymer), strengths, and weaknesses. Some of these are in development or undergoing preclinical evaluation, while others have already been implanted in humans. However, the interplay between mechanical dilation, resorption, and arterial response following implantation of bioresorbable scaffolds is still poorly understood [Strandberg E et al. Circ Cardiovasc Interv 2012].
Onuma et al. [Circ J 2011] reported that the current generation of BRS is composed of either a polymer or a bioresorbable metal alloy. Numerous polymers are available, each with different chemical compositions, mechanical properties, and, consequently, bioabsorption times.
The most frequently used polymer in the current generation of BRS is poly-L-lactic acid (PLLA), which is already in widespread clinical use, with applications such as resorbable sutures, soft tissue implants, orthopedic implants, and dialysis media [Onuma Y, Serruys PW. Circulation 2011].
The Bioresorption Process of Poly-L-Lactide
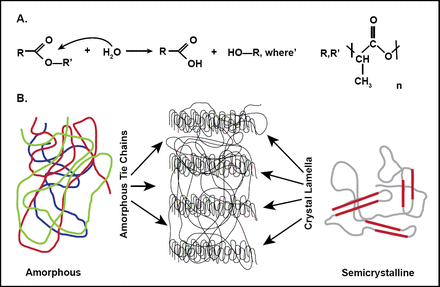
In the polylactic acid (PLA) family of polymers, molecular-weight degradation occurs in vivo mainly through hydrolysis, a bimolecular nucleophilic substitution reaction that can be catalyzed by the presence of either acids or bases [Onuma Y, Serruys PW. Circulation 2011]. Figure 1 shows the hydrolysis reaction in which water catalyzes a chain scission event at an ester bond.
Polyactide Degradation Mechanism
Reproduced from Onuma Y and Serruys PW. Bioresorbalbe Scaffold: The advent of a new era in Percutaneous coronary and peripheral revascularization. Circulation 2011; 7(123): 779, with permission from the American Heard Association.
PLLA is a semicrystalline polymer. Ordered polymer chains constitute the crystalline component of the semicrystalline polymer, and random ones form the amorphous segment [Onuma Y, Serruys PW. Circulation 2011]. The properties of semicrystalline polymers make them well-suited for mechanical support (eg, the scaffold). Amorphous polymers allow for a more uniform dispersion of the drug, and are therefore used in controlled drug release systems (eg, coating of the bioresorbable vascular scaffold system) [Onuma Y et al. Circ J 2011].
From a chemical standpoint, resorbable implants undergo 5 stages that are not discrete and can overlap. Onuma and Serruys [Circulation 2011] reported that the first stage is hydration of the polymer. After implantation of the polymeric resorbable device, the polymers begin to absorb water from the surrounding tissue. The second phase is depolymerization by hydrolysis. This is first observed by a reduction in molecular weight. The loss of mass (third stage) occurs when the implant no longer has cohesive strength and the polymer starts to fragment into segments of low-weight polymer. The fourth phase is assimilation or dissolution of the monomer. Lastly, the soluble monomer (eg, L-lactate) is changed into pyruvate, which eventually enters the Krebs cycle and is converted into carbon dioxide and water. These final products are excreted from the body through the kidneys or lungs, which results in complete bioresorption of the implant.
Key Points
Based on data from recent research, Prof. Serruys made several key points. One message was that PLLA fully disappears after 2 years. According to a study using optical coherence tomography (OCT) and histology in a porcine coronary artery model, struts that were still discernible by OCT at 2 years were compatible with largely bioresorbed struts. At 3 and 4 years, both OCT and histology confirmed complete integration of the struts into the arterial wall [Onuma Y et al. Circulation 2010].
He noted that bioresorption is a real phenomenon. In an assessment of the safety of the bioabsorbable everolimus-eluting stent, Prof. Serruys and colleagues found that 2 years after implantation, the stent was bioabsorbed, vasomotion was restored, and restenosis was prevented in the absence of a late safety signal, suggesting freedom from late thrombosis [Serruys PW et al. Lancet 2009].
Other data show that the impact of physiological cyclic strain and shear stress are essential for vessel wall biology. Hahn and Schwartz [Nat Rev Mol Cell Biol 2009] found that forces associated with blood flow are major determinants of vascular morphogenesis and physiology. Their review on mechanotransduction in vascular physiology and atherogenesis demonstrated that blood flow is crucial for blood vessel development during embryogenesis and for regulation of vessel diameter in adult life. It is also a key factor in atherosclerosis, which, despite the systemic nature of major risk factors, occurs mainly in regions of arteries that experience disturbances in fluid flow.
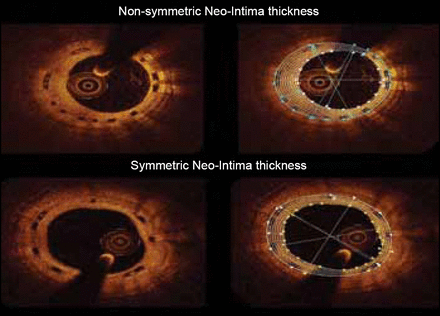
Brugaletta et al. [Atherosclerosis 2012] quantified the circumferential healing process by OCT at 6 and 12 months following the implantation of the ABSORB bioresorbable vascular scaffold. A total of 58 patients (59 lesions) were included in the analysis. The neointima area was not different between 6 and 12 months follow-up (1.57±0.42 vs 1.64±0.77 mm2; p=0.691). There was also no difference in mean thickness of the neointima (median [interquartile range]) between the 2 follow-up time points (210 μm [180 to 260] vs 220 μm [150 to 260]; p=0.904). However, the symmetry of the neointima thickness was higher at 12 months follow-up than at 6 months (0.23 [0.13 to 0.28] vs 0.16 [0.08 to 0.21]; p=0.019).
According to Prof. Serruys, the implantation of bioabsorbable scaffolding not only seals and shields plaques, but it also caps them for up to 60 months of follow-up. Other benefits include wall thinning and plaque/media reduction out to 5 years of follow-up with OCT showing late lumen enlargement (Figure 2).
Symmetry of Neointima at 6 and 12 months
Reproduced from Brugaletta S et al. Circumferential evaluation of the neointima by optical coherence tomography after ABOSRB bioresorbable vascular Scaffold Implantation Atherosclerosis 2012; 221(1):106–112, with permission from Elsevier.
- © 2012 MD Conference Express®