Summary
Hepatitis C virus (HCV) has infected at least 170 million people worldwide and has superseded HIV as a cause of death in the United States. HCV is a major cause of liver disease, but therapeutic options are limited and there is no vaccine. New policy initiatives to detect patients with chronic hepatitis and more effective antivirals are crucially needed. This article discusses emerging issues in the management of hepatitis C infection.
- Emerging Therapies
- Viral Infections
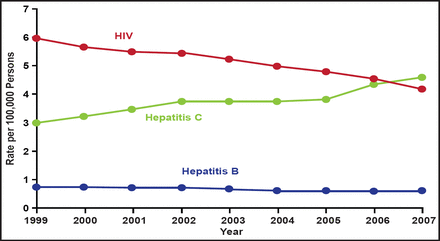
Hepatitis C virus (HCV) has infected at least 170 million people worldwide and has superseded HIV as a cause of death in the United States (Figure 1). HCV is a major cause of liver disease, but therapeutic options are limited and there is no vaccine. New policy initiatives to detect patients with chronic hepatitis and more effective antivirals are crucially needed. In his keynote address, Charles M. Rice, PhD, Rockefeller University, New York, New York, USA, discussed emerging issues in the management of hepatitis C infection.
Age-Adjusted Hepatitis C Mortality Surpasses HIV in the United States, 1999 to 2007.
Ly KN. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Ann Intern Med. 21 February 2012;156(4):271–278 with permission from the American College of Physicians.
Despite 20 years of HCV research, challenges remain in the areas of both drug and vaccine development. Part of the difficulty in deriving new antiviral treatment for HCV is its genetic diversity, but even with the more than 30% nucleotide sequence divergence between genotypes, HCV variants remain remarkably similar in their transmission dynamics, persistence in the face of host-cell defenses and the immune system, and disease development. In addition, while the mutability and large population size of HCV enables it to respond very rapidly to new selection pressures, its ability to diversify is constrained by its intimate adaptation to its host, the human liver [Simmonds P. J Gen Virol 2004].
The goals for HCV research are to clean up the blood supply (which has been achieved in many countries), to educate (and possibly develop a vaccine for) high-risk groups, to identify infected individuals, and to develop improved treatments—with the ultimate goal of eradicating the virus. Important to the achievement of these goals was the new development in 2005 of a robust cell culture (HCVcc) that reflects the entire life cycle of HCV. With this culture it is now possible to replicate the virus in cell culture without adaptive mutations. A great deal has been learned about the life cycle of this RNA virus since the development of the new culture system.
Entry Factors at Hepatocytes
Viral entry is the first step of infection and requires the cooperative interaction of several host cell factors, which include low-density lipoproteins, very-low-density lipoproteins (VLDL), and the apolipoproteins E and C1. HCV assembly and maturation occur in the endoplasmic reticulum (ER) and post-ER compartments, respectively. By co-opting the VLDL assembly, maturation, and secretory machinery of the cell, HCV acquires its hepatocyte tropism and, by mimicry, its tendency to persist [Gastaminza P et al. J Virol 2008]. Thus viral entry represents a potential multifaceted target for antiviral intervention.
In a recent study Sainz and colleagues [Nat Med 2012] showed that the cellular Niemann-Pick C1-Like 1 (NPC1L1) cholesterol uptake receptor is necessary for HCV infection, as silencing or antibody-mediated blocking of NPC1L1 impairs HCVcc infection initiation. In addition the NPC1L1 antagonist ezetimibe, which potently blocks HCV uptake, inhibits infection by all major HCV genotypes in vitro. Epidermal growth factor receptor and ephrin receptor A2 have also been identified as host cofactors for HCV entry. Blocking receptor kinase activity broadly impairs infection by all major HCV genotypes and viral escape variants in cell culture and in a human liver chimeric mouse model in vivo [Lupberger J et al. Nat Med 2011].
Translation and Replication
The current HCV therapy of pegylated interferon (PegIFN) plus ribavirin (RBV) is poorly tolerated and frequently ineffective, so there is an urgent need for new drugs. miR-122 plays an important positive role in the regulation of HCV replication by binding directly to 2 adjacent sites close to the 5' end of HCV RNA. It can selectively and effectively be inhibited with antisense oligonucleotides. Miravirsen, an antisense oligonucleotide, is undergoing Phase 2a clinical trials in a group of chronic HCV genotype 1 infection patients. Another promising area of drug development is direct-acting antivirals targeting at 3 replication proteins: the NS3–4A serine protease; the NS5B RNA-dependent RNA polymerase; and NS5A, a multifunctional protein involved in HCV RNA replication and virus assembly. Together these 3 targets are the guts of the RNA replication machine.
An interesting facet of this disease is that most people do not know they are infected. It may take weeks or even months before the immune system wakes up and attempts to fight the infection. In acute infection, 30% of those infected recover and 70% go on to develop chronic infection, which can lead to cirrhosis, liver cancer, and end-stage liver disease (ESLD). The inability to predict which patients are going to progress to ESLD is frustrating for both patients and physicians. Treatment of all infected patients could reduce the risk of cirrhosis (16%), decompensation (42%), cancer (31%), and liver-related deaths (36%) by 2020, given current response rates to antiviral therapy if all infected patients could be identified. Unfortunately, less than 50% of infected patients have been identified and even fewer treated [Davis GL et al. Gastroenterology 2010].
The good news regarding HCV is that successful treatment means cure in a vast majority of cases. A successful treatment is defined as a sustained virologic response (SVR), ie, being HCV negative 6 months after cessation of treatment. With the elimination of the virus there is a lower risk of liver disease and cancer. Until 2011, the most effective approved treatment was the combination of PegIFN and RBV for 48 weeks. In mid 2011, 2 protease inhibitors (boceprevir and telaprevir) were approved in combination with the previous standard of care, resulting in SVR rates of over 70% for HCV patients infected with genotype 1 [Jacobson IM et al. New Engl J Med 2011; Poordad F et al. New Engl J Med 2011].
Dr. Rice commented that the ultimate goal for treatment is to have an effective oral, IFN/RBV-free HCV therapy. Currently the cure rate is about 70% to 75% and many of the new compounds have limited genotype coverage. Resistance and side effects (which often lead to hospitalization) are still problematic. The cost of treatment must also be reduced since the drugs need to have worldwide accessibility.
Treatment is going to get more complicated before it gets simple, as there are more than 50 compounds in clinical development. While the future is bright for HCV treatment, the question remains as to whether a preventative vaccine will be needed to achieve global control and, ultimately, eradication of this widespread disease.
- © 2012 MD Conference Express®