Summary
The first human transcatheter aortic valve implantation (TAVI) was performed in 2002. Since then, TAVI has been evaluated as an alternative to surgical aortic valve replacement and medical treatment in several clinical trials. This article reviews the evidence from these trials. Also discussed are current tools for risk stratification and the risks of TAVI in low- and high-risk patients, and latest guidelines on the management of valvular heart disease.
- Interventional Techniques & Devices
- Valvular Disease
Lessons from Clinical Trials
The first human transcatheter aortic valve implantation (TAVI) was performed in 2002. Since then, TAVI has been evaluated as an alternative to surgical aortic valve replacement (SAVR) and medical treatment (MT) in several clinical trials. Steven Windecker, MD, Bern University Hospital, Bern, Switzerland, reviewed the evidence from these trials.
The Placement of Aortic Transcatheter Valve [PARTNER] B trial, assessed TAVI versus MT in inoperable patients with symptomatic aortic stenosis (AS) [Leon MB et al. N Engl J Med 2010; Makkar RR et al. N Engl J Med 2012]. TAVI versus standard treatment (including balloon aortic valvuloplasty) resulted in lower rates of all-cause death (43.3% vs 68.0%; HR, 0.56; 95% CI, 0.43% to 0.73%; p<0.001) and cardiac death (31.0% vs 62.4%; HR, 0.44; 95% CI, 0.32% to 0.60%; p<0.001) at 2 years. Cerebrovascular event rates were 13.8% with TAVI versus 5.5% with MT (HR, 2.79; 95% CI, 1.25% to 6.22%; p=0.01). The rate and severity of paravalvular aortic regurgitation (AR) with TAVI decreased from 30 days to 2 years after implantation (p=0.001), while transvalvular AR rates did not change significantly (p=0.75). Mortality rates were higher in patients with AR (p<0.01).
In the PARTNER A trial of TAVI versus SAVR in high-risk patients with symptomatic AS, all-cause death rates were similar after at least 2 years follow-up (HR, 0.90; 95% CI, 0.71% to 1.15%; p=0.41) [Smith CR et al. N Engl J Med 2011; Kodali SK et al. N Engl J Med 2012]. Stroke rates were 7.7% with TAVI versus 4.9% with SAVR (HR, 1.22; 95% CI, 0.67% to 2.23%; p=0.52), as was paravalvular regurgitation (p<0.001). After 2 years, aortic valve area and mean aortic gradient were similar in the TAVI and SAVR groups (p=0.16 for both).
During the past decade, clinical trials have shown that TAVI is superior to standard treatment and non-inferior compared with SAVR. However, stroke is an issue early after TAVI. Valve durability appears to be maintained beyond 2 years of follow-up, but the impact of AR on outcomes needs to be improved. TAVI effectively alleviates symptoms and improves health-related quality of life (QoL) compared with standard medical therapy.
Low- and High-Risk Patients: What Are the Limits?
Bernard Prendergast, DM, John Radcliffe Hospital, Oxford, United Kingdom, discussed patient selection for TAVI. He reviewed current tools for risk stratification and the risks of TAVI in low- and high-risk patients.
The PARTNER trial had strict entry criteria, requiring 2 cardiac surgeons and an interventionist to attest that candidates were not suitable for SAVR (high surgical risk due to coexisting conditions associated with mortality risk of at least 15% by 30 days after surgery [Society of Thoracic Surgeons (STS) score ≥10%]) [Leon MB et al. N Engl J Med 2010]. In a registry of 3195 high-risk TAVI patients, 835 (26%) had a European System for Cardiac Operative Risk Evaluation (EuroSCORE) <20% and an STS score <10% but had contraindications to surgery [Gilard M et al. N Engl J Med 2012]. Lange et al. [J Am Coll Cardiol 2012] reported that clinical outcomes were significantly better in lower-versus higher-risk patients undergoing TAVI in a single center from 2007 to 2010.
In another observational study, patients allocated to SAVR were younger and had lower predicted perioperative risk than patients allocated to MT or TAVI [Wenaweser P et al. J Am Coll Cardiol 2011]. At 30 months, mortality was lower with SAVR (22.4%) and TAVI (22.6%) compared with MT (61.5%; p<0.001).
According to Kovac et al. [Heart 2010], the EuroSCORE and STS scores have not been well validated in estimating risk for valve-only surgery; some TAVI-specific measures are not considered, and social and QoL issues are not included. Van Brabandt et al. [BMJ 2012] claimed that TAVI is risky and costly, with rapid expansion in Europe beyond the evidence base and with insufficient device regulation. Concerns presented by Van Brabandt and colleagues included the cost and the learning curve associated with these new procedures. In addition they criticized the PARTNER trial because cost-effectiveness data had not been published, benefits in the subgroup undergoing TAVI by a transapical approach were less clear, and there were limitations in matching the treatment groups in Cohort B.
Overall, Prof. Prendergast recommended that TAVI be considered in patients with indications for AVR who are high-risk for surgery as described by clinical trials and guidelines. In addition, he noted that it is important to consider which patients are less likely to benefit from TAVI, including those with a EuroSCORE >40, severe left or right ventricle impairment, severe respiratory disease, severe immobility, or life expectancy <1 year. Patients with EuroSCORE <10 are too low-risk to be considered for TAVI, especially if TAVI selection is driven by patient choice. Assessment by a comprehensive heart team, as was performed in the clinical trials, is strongly recommended to determine patient suitability for TAVI.
Guidelines for Management of Severe AS
Bernard Iung, MD, Hôpital Bichat, Paris, France, reviewed the latest guidelines on the management of valvular heart disease. The European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines recommend evaluating severity of disease, presence of symptoms, life expectancy, QoL, intervention benefits versus risks, patient wishes, and available resources. Severe AS is defined as aortic valve area (AVA) <1.0 cm2, indexed AVA <0.6 cm2, mean gradient >40 mm Hg, maximum jet velocity >4.0 m/s, and velocity ratio <0.25 [Vahanian A et al. Eur Heart J 2012]. While the guidelines do provide the listed criteria, these parameters have been shown to perform inconsistently in patients with normal LV function in whom use of AVA criteria results in a greater proportion of patients being classified as having severe AS [Minners J et al. Eur Heart J 2008]. These inconsistencies should be considered when evaluating patients with AS.
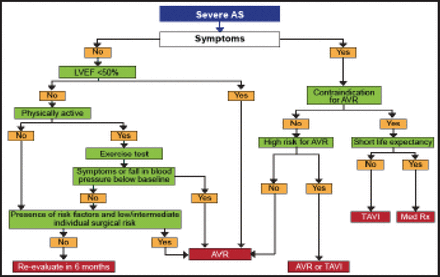
The guidelines recommend TAVI for eligible patients with contraindications to surgical AVR [Vahanian A et al. Eur Heart J 2012] (Figure 1). In the PARTNER trials, EuroSCOREs and STS scores were 26.4% and 11.2%, respectively, in patients with contraindications for surgery [Leon MB et al. N Engl J Med 2010] and 29.3% and 11.8% in high-risk operable patients [Smith CR et al. N Engl J Med 2011]. Risk scores have good discrimination (low- vs high-risk) but poor calibration (predicted vs observed risk). EuroSCORE II has improved calibration, but there are no specific data in high-risk patients. The ESC Working Group reported that risk scores have limitations in high-risk patients and that patients with comorbidities require an individualized approach. In the absence of a more precise quantitative score, risk assessment should mostly rely on the clinical judgment of a comprehensive heart team as was used in the PARTNER trials [Leon MB et al. N Engl J Med 2010; Makkar RR et al. N Engl J Med 2012].
Management of Severe AS.
AS=aortic stenosis; AVR=aortic valve replacement; LVEF=left ventricular ejection fraction; TAVI=transcatheter aortic valve implantation.
Reproduced with permission from the European Society of Cardiology. All rights reserved. Copyright © 2012.
TAVI should be considered for patients with severe symptomatic AS who are deemed to be high-risk for traditional SAVR. Risk assessment is a key issue, and a better definition of contraindications to SAVR is needed. Risk scores in patients with AS have limitations, and there is a need for better identification of patients who should not have any intervention. Clinical judgment through a multidisciplinary approach is essential for optimal patient selection.
TAVI in the “Real World”
Based on a review of data from TAVI registries, Martyn R. Thomas, MD, St. Thomas' Hospital, London, United Kingdom, concluded that TAVI is rapidly being adopted in the real world but penetration varies widely from country to country. Outcome results for the Sapien and CoreValve devices are the same except for higher permanent pacemaker rates with the CoreValve. Mortality is 4% to 8% at 30 days and 15% to 25% at 1 year after TAVI, and stroke rates are 3% to 4% [Toggweiler S et al. J Am Coll Cardiol 2012; Wendler O. TCT 2012; Meridith IT. TCT 2010]. Moderate to severe paravalvular leak is associated with worse outcomes, as was shown in the PARTNER trials. The registries contain only limited QoL data. Comparison between transfemoral and alternative access outcomes is difficult because the patient populations are different, as shown in the SOURCE XT registry (Table 1).
TF Versus Non-TF: SOURCE XT.
Overall, novel interventional options for patients with AS who need AVR continue to evolve. These therapies provide an opportunity to improve patient outcomes. Importantly, adoption of these new therapies should be based primarily on clinical outcome data rather than enthusiasm, finances, and healthcare systems. Optimal outcomes can only be achieved through sensible use governed by current data and caution should be used with regard to performing TAVI in lower-risk patients.
- © 2012 MD Conference Express®