Summary
For patients with a history of myocardial infarction (MI) who are stable, the addition of vorapaxar to the standard of care reduced the long-term risk of cardiovascular death or ischemic events and increased the risk of moderate or severe bleeding. The efficacy and safety of the drug were evaluated for secondary prevention in a broad group of patients with prior MI, prior stroke, or peripheral artery disease in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events-Thrombolysis in Myocardial Infarction 50 [TRA 2°P-TIMI 50] trial.
- Thrombotic Disorders
- Myocardial Infarction
- Coronary Artery Disease
- Cerebrovascular Disease
- Cardiology Clinical Trials
For patients with a history of myocardial infarction (MI) who are stable, the addition of vorapaxar to the standard of care reduced the long-term risk of cardiovascular (CV) death or ischemic events and increased the risk of moderate or severe bleeding. These findings are among the first to show a benefit of adding intense antiplatelet treatment to standard therapies for long-term secondary prevention in patients with a history of MI.
Vorapaxar inhibits platelet activation by antagonizing thrombin-mediated activation of the protease activated receptor-1 (PAR-1) expressed on platelets. The efficacy and safety of the drug were evaluated for secondary prevention in a broad group of patients with prior MI, prior stroke, or peripheral artery disease in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events-Thrombolysis in Myocardial Infarction 50 [TRA 2°P-TIMI 50] trial. Benjamin M. Scirica, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the primary results in the subgroup of patients who were randomized with a qualifying MI (type 1 MI within the previous 2 weeks to 12 months), with publication timed to coincide with the presentation at the European Society of Cardiology Congress 2012 [Scirica BM et al. Lancet 2012].
The MI subgroup included 17,779 patients who had a qualifying spontaneous MI; 8898 were randomly assigned to vorapaxar and 8881 to placebo. A low–bleeding-risk cohort was identified (n=14,909). The primary efficacy endpoint was CV death, MI, or stroke analyzed by intention to treat. The principal safety endpoint was Global Use of Strategies to Open Occluded coronary arteries (GUSTO) moderate or severe bleeding. Most (98%) patients were on aspirin and 78% were on a thienopyridine. The median follow-up was 30 months, and the results were presented as 3-year Kaplan-Meier estimates.
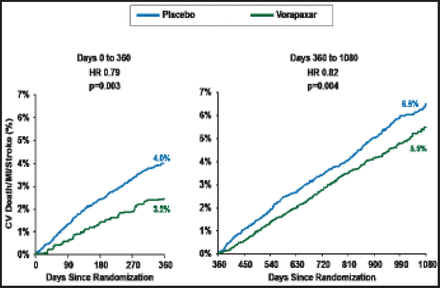
Dr. Scirica reported that vorapaxar significantly reduced the rate of the primary endpoint compared with placebo (8.1% vs 9.7%; HR, 0.80; 95% CI, 0.72 to 0.89; p<0.0001). Vorapaxar reduced the rate of the primary endpoint both early (Day 0 to 360; p=0.003) and late (Day 360 to 1080; p=0.004; Figure 1).
Early and Late Efficacy of Vorapaxar Among the Subgroup of Patients with a Prior MI Cohort.
Reproduced with permission from The Lancet; Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: A prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Scirica BM et al. 2012;doi:10.1016/S0140–6736(12)61269–0.
Based on prior studies, a cohort of patients (n=14,909; 84% of the MI cohort) who were age <75 years, had no history of TIA or stroke, and were ≥60 kg were selected as having the best potential for net clinical benefit [Wiviott SD et al. N Engl J Med 2007]. In this group, CV death, MI, or stroke was less common in the vorapaxar group compared with those in the placebo group (6.8% vs 8.6%; HR, 0.75; 95% CI, 0.66 to 0.85; p<0.0001), as was CV death (1.5% vs 2.0%; HR, 0.73; 95% CI, 0.56 to 0.95; p=0.020).
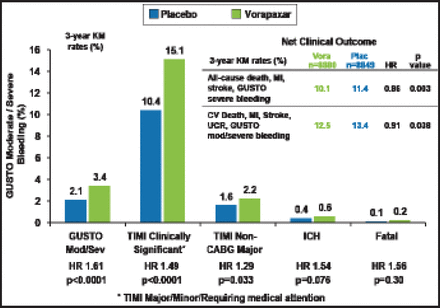
Consistent with the overall trial safety results, vorapaxar increased GUSTO moderate or severe bleeding (3.4% vs 2.1%; p<0.0001), as well as TIMI clinically significant bleeding (15.1% vs 10.4%; p<0.0001); TIMI major bleeding not associated with coronary artery bypass grafting (2.2% vs 1.6%; p=0.033; Figure 2). Rates of intracranial hemorrhage (0.6% vs 0.4%) and fatal bleeding (0.2% vs 0.1%) were not numerically higher but significantly different (p=0.076 and p=0.30, respectively).
Bleeding Endpoints.
CV=cardiovascular; GUSTO=Global Use of Strategies to Open Occluded coronary arteries; ICH-intracranial hemorrhage; KM=Kaplan-Meier; TIMI=Thrombolysis in Myocardial Infarction; UCR-urgent coronary revascularization.
Reproduced with permission from BM Scirica, MD.
The net clinical benefit in the MI cohort with vorapaxar was significant, with a lower rate of all-cause death, MI, stroke, and GUSTO severe bleeding (10.1% vs 11.4%; HR, 0.86; p=0.003) and a lower rate of CV death, MI, stroke, urgent revascularization, and GUSTO moderate/severe bleeding (12.5% vs 13.4%; HR, 0.91; p=0.038). Dr. Scirica noted that the benefit of vorapaxar was consistent, offering an advantage regardless of the timing of MI, at early and late time periods, and with or without use of a thienopyridine in addition to aspirin.
- © 2012 MD Conference Express®