Summary
In about half of all patients with heart failure (HF), the ejection fraction (EF) is normal or nearly normal, despite high morbidity and mortality in these patients. Now, the findings of a Phase 2 trial suggest that a first-in-class angiotensin receptor neprilysin inhibitor, LCZ696, may be beneficial for patients who have HF with preserved EF. This article discusses the Prospective Comparison of ARNI with ARB (angiotensin receptor blocker) on Management of Heart Failure with Preserved Ejection Fraction [PARAMOUNT; NCT00887588] trial.
- Cardiology Clinical Trials
- Heart Failure
In about half of all patients with heart failure (HF), the ejection fraction (EF) is normal or nearly normal, despite high morbidity and mortality in these patients. Now, the findings of a Phase 2 trial suggest that a first-in-class angiotensin receptor neprilysin inhibitor (ARNI), LCZ696, may be beneficial for patients who have HF with preserved EF.
LCZ696 is the first agent to be associated with 2 powerful predictors of outcome in HF: reduction in the concentration of N-terminal prohormone brain natriuretic peptide (NT-proBNP) and decrease in the size of the left atrium, said Scott D. Solomon, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, who reported the findings. The study was published to coincide with its presentation at the ESC 2012 Congress [Solomon SD et al. Lancet 2012].
Dr. Solomon explained that LCZ696 simultaneously blocks the renin angiotensin system while augmenting the body's intrinsic natriuretic peptide system through neprilysin inhibition. These dual effects may be important in the treatment of HF with preserved EF.
The Prospective Comparison of ARNI with ARB (angiotensin receptor blocker) on Management of Heart Failure with Preserved Ejection Fraction [PARAMOUNT; NCT00887588] trial enrolled 308 patients who were randomly assigned to LCZ696 (200 mg BID after 1 week each of 50 and 100 mg BID) or the ARB valsartan (160 mg BID after 1 week each of 40 and 80 mg BID). The primary endpoint, NT-proBNP, was evaluated as the ratio of the concentration at 12 weeks to that at baseline. Secondary objectives included echocardiographic measures of left atrial size, left ventricular size and function, and diastolic function, and safety and tolerability.
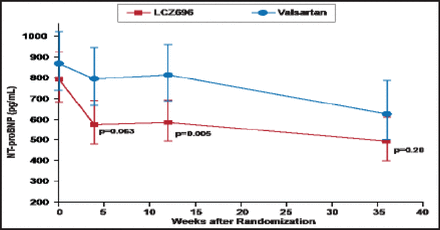
Over 12 weeks, both LCZ696 and valsartan led to a decrease in the NT-proBNP concentration, with a greater reduction in the LCZ696 group (from 783 to 605 pg/mL) than in the valsartan group (from 862 to 835 pg/mL; HR, 0.77; 95% CI, 0.64 to 0.92; p=0.005). The decrease was evident at 4 weeks and was sustained over the 12-week period (Figure 1). The NT-proBNP concentration continued to decrease in both groups over 36 weeks; the difference no longer significant at that time (p=0.20).
Comparison of the Primary Endpoint—Concentration of NT-proBNP at 12 Weeks.
Reproduced with permission from The Lancet; The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Solomon SD et al. 2012;doi:10.1016/S0140–6736(12)61227–6.
A similar treatment effect was found in all predefined subgroups, including those defined by age (≥65 vs <65 years), gender, systolic blood pressure (>140 vs ≤140 mm Hg), presence or absence of diabetes, EF (≥50% vs <50%), presence or absence of atrial fibrillation, previous hospitalization for HF, NYHA class (III vs II), and median NT-proBNP concentration (>median vs ≤median).
LCZ696 was also associated with a significant decrease in the volume of the left atrium at 36 weeks (p=0.003), and the left atrial dimension (p=0.034). In addition, the NYHA class improved in more patients in the LCZ696 group than in the valsartan group at both 12 and 36 weeks; the difference was significant at the latter time period (p=0.05). The frequency of serious adverse events was similar between therapies: 15% with LCZ696 and 20% with valsartan. The number of patients with hypotension, renal dysfunction, or hyperkalemia did not differ between groups.
Prospective studies are needed to determine whether the effects found in PARAMOUNT translate into improved clinical outcomes.
- © 2012 MD Conference Express®