Summary
Insulin is released continuously by the pancreas at a nearly constant rate between meals and in the fasting state (basal insulin secretion). This article discusses the data on various basal insulins, as well as evolution of rapid-acting insulin, limitations of current insulin preparations, and pipeline products.
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Insulin
Basal Insulin
Insulin is released continuously by the pancreas at a nearly constant rate between meals and in the fasting state (basal insulin secretion). The pivotal role of basal insulin is to restrain the release of glucose from the liver and free fatty acids from adipose tissue, thus preventing hyperglycemia and ketosis [Bolli GB et al. Diabetes Technol Ther 2011]. Geremia B. Bolli, MD, University of Perugia, Perugia, Italy, presented data on various basal insulins.
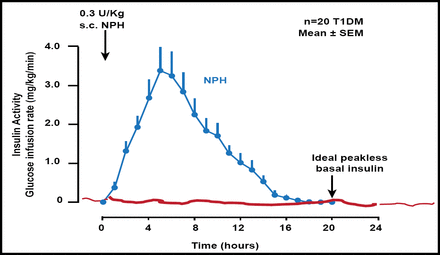
Neutral protamine Hagedorn (NPH) insulin and NPH-based insulin mixtures have early peak effects and a relatively short duration of action. They carry a risk of nocturnal hypoglycemia and fasting hyperglycemia, respectively, after the evening injection [Bolli GB et al. Diabetes Technol Ther 2011] (Figure 1).
NPH and NPH-Based Insulin Mixtures Have Early Peak Effects and Relatively Short Duration of Action.
Reproduced with permission from GB Bolli, MD.
Compared with NPH insulin, glargine provides greater metabolic activity and superior glucose control for up to 32 hours [Lucidi P et al. Diabetes Care 2011]. Glargine is a peakless insulin that lasts nearly 24 hours, has lower intersubject variability than NPH insulin, and closely mimics continuous subcutaneous insulin infusion (CSII). The gold standard means of basal insulin replacement [Lepore M et al. Diabetes 2000], CSII delivers rapid-acting insulin that mirrors the endogenous insulin pattern of the pancreas.
Only long-acting analogs, such as glargine (>24 hours in duration, once a day) and detemir (<24 hours in duration, once or twice a day), should be used as a basal insulin in type 1 diabetes in combination with mealtime rapid-acting analogs [Bolli GB. Diabetes Technol Ther 2011]. The preferred means of delivery is CSII.
Detemir is associated with a higher insulin dose compared with insulin glargine across a wide body mass index range [Holleman F et al. Diabetes 2011]. CSII is a superior option, owing to better reproducibility or subcutaneous absorption of soluble insulin. Although CSII is not superior to multiple daily insulin injections in the general population of people with type 1 diabetes, it might be indicated in subsets of those with type 1 diabetes [Bolli GB et al. Diabetes Technol Ther 2011].
Basal insulin degludec, which is still under development, is an ultra-long-acting basal insulin [Heller S. Lancet 2012]. Its pharmacokinetic profile predicts a sustained effect (>24 hours), low variability in subcutaneous absorption, and a low rate of hyperglycemia. Day-to-day variability in glucose-lowering effect is 4 times lower with degludec versus glargine [Heise T et al. Diabetes Obes Metab 2012].
A novel basal insulin that is under development, LY2605541, is engineered to be large in size, thereby delaying insulin absorption, reducing clearance, and resulting in prolonged duration of action [Hansen RJ et al. ADA 2012 Abstract 896-P]. Questions that remain on LY2605541 include its pharmacokinetic profile in type 1 diabetes, day-to-day reproducibility, lipid/hepatic mechanism and weight loss in the face of improved glycemic control, and titration.
Dr. Bolli said the replacement of basal insulin is fundamental to insulin treatment of types 1 and 2 diabetes, and substitution of basal insulin becomes increasingly more important and challenging as β-cell mass or function deteriorates over time.
Newer Bolus Insulins
Luigi F. Meneghini, MD, University of Miami Miller School of Medicine, Miami, Florida, USA, discussed the evolution of rapid-acting insulin, limitations of current insulin preparations, and pipeline products.
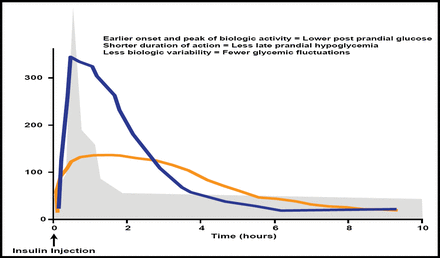
Home et al. recently reported [Diabetes Obes Metab 2012] that postprandial glucose excursions can inhibit achievement of good glycemic control and possibly have an effect on the risk of vascular comorbidities. Rapid-acting analogs offer an earlier onset and peak of biologic activity, a shorter duration of action, and less biologic variability (Figure 2), potentially leading to more physiologic insulin insulin replacement and better overall control than with human insulin.
Potential Advantages of Rapid-Acting Insulin Analogs.
Reproduced with permission from LF Meneghini, MD.
A Cochrane review of the effects of short-acting insulin analogs versus regular insulin, however, suggested only a modest benefit of short-acting insulin analogs in the majority of patients treated with insulin [Siebenhofer A et al. Cochrane Database Syst Rev 2006]. Clearly the timing of insulin bolus to meal is an important determinant of post-prandial glycemic control, and should favor rapid-acting insulin analogs. It is clear, from a study on the optimal timing of bolus administration in relation to meal consumption in adolescents and adults with type 1 diabetes, that a bolus of rapid-acting insulin prior to a meal results in significantly better postprandial glucose control than when the meal insulin bolus is given just prior to the meal or 20 minutes after meal initiation. [Cobry E et al. Diabetes Technol Ther 2010].
Insulin analogs are absorbed more quickly than human insulin in normal-weight healthy subjects or lean subjects with type 1 diabetes. However, most patients with type 2 diabetes using insulin are distinctly overweight or obese and require much larger insulin dosages [Lane WS et al. Endocr Pract 2009]. Gagnon-Auger et al. [Diabetes Care 2010] found that absorption of even a rapid-acting insulin analog such as lispro is substantially delayed and biologic activity considerably lower in obese subjects with type 2 diabetes.
There are a number of innovative technologies being applied to provide a more physiologic insulin action profile. One such approach is the use of a fully human recombinant DNA-derived hyaluronidase enzyme (rHuPH20), which reduces resistance to bulk fluid flow following drug injection (insulin) and promotes drug dispersion to allow exposure to a greater capillary bed area. This approach accelerates absorption and peak biologic activity, as well as shortens the duration of action of either regular insulin or rapid-acting insulin analog preparations. [Frost GI. Expert Opin Drug Deliv 2007].
Another approach—inhaled insulin delivery—is generally well-tolerated and appears to be safe, at least in the short term [Muchmore DB, Gates JR. Diabetes Obes Metab 2006]. Inhaled insulin offers an alternative noninvasive option for premeal insulin administration, with glycemic efficacy slightly less than subcutaneous regular insulin and increased acceptability [Ceglia L et al. Ann Intern Med 2006].
A randomized, open-label, parallel-group study [NCT00309244] to assess the efficacy and safety of prandial Technosphere® inhaled insulin compared with twice-daily biaspart insulin found that change in HbA1C with inhaled insulin plus insulin glargine (0.68%; SE, 0.077; 95% CI, 0.83 to 0.53) was similar and noninferior to that with biaspart insulin (0.76%; SE, 0.071; 0.90 to 0.62).
Patients had significantly less weight gain (p<0.05) and a reduced incidence of mild-to-moderate and severe hypoglycemic events on inhaled insulin plus insulin glargine than on biaspart insulin. The safety and tolerability profile was similar for both treatments, although there was an increased occurrence of cough and change in pulmonary function in the group that received inhaled insulin plus insulin glargine [Rosenstock J et al. Lancet 2010]. Concerns with respect to chronic lung exposure of high insulin concentrations still need to be carefully evaluated.
- © 2012 MD Conference Express®