Summary
Experimental data suggest that the novel Trevo device is highly effective at achieving immediate reperfusion of occluded arteries without causing any clinically significant disruption of vascular integrity [Nogueira RG et al. J Neurointerv Surg 2011]. This article also reports on the promising findings from the Phase 4 Thrombectomy REvascularization of Large Vessel Occlusions in Acute Ischemic Stroke trial [TREVO; NCT01088672].
- Valvular Disease
- Interventional Techniques & Devices Clinical Trials
- Cerebrovascular Disease
- Ischemia
- Cerebrovascular Disease
Experimental data suggest that the novel Trevo device is highly effective at achieving immediate reperfusion of occluded arteries without causing any clinically significant disruption of vascular integrity [Nogueira RG et al. J Neurointerv Surg 2011]. Nils Wahlgren, MD, PhD, Karolinska Institutet, Stockholm, Sweden, also reported promising findings from the Phase 4 Thrombectomy REvascularization of Large Vessel Occlusions in Acute Ischemic Stroke trial [TREVO; NCT01088672].
This multicenter, international, prospective, single-arm clinical trial included 60 patients at 7 sites in Germany, Spain, Austria, and Sweden. The procedure employs a microcatheter that is placed distal to the thrombus to deliver the Trevo device, which is deployed by unsheathing the microcatheter and allowing clot integration into the device. The Trevo is then retrieved into a proximally placed catheter.
The purpose of the study was to determine the revascularization rate of the Trevo system in large-vessel occlusions in ischemic stroke patients. The primary endpoint was revascularization, defined as at least thrombolysis in cerebral infarction (TICI) score 2a. Secondary endpoints included modified Rankin scale (mRS) clinical outcomes at 90 days; mortality at 90 days; device-related serious adverse events, as determined by an independent clinical events committee; and symptomatic intracranial hemorrhage (sICH) rate within 24 hours according to Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria, as determined by an independent core lab [Wahlgren N et al. Lancet 2007]. Neuroimaging (CT or MR) was required at 24 hours. Clinical evaluation also took place at 7 and 90 days.
The median patient age was 65 years, with a range from 21 to 84 years; 45% of the patients were male. Atrial fibrillation accounted for most of the strokes (41.7%), followed by unknown cause (25%), large-artery atherosclerosis (20%), other- cardioembolic (8.3%), and other (3.3%). The median National Institute of Health Stroke Score (NIHSS) was 18, with a range of 8 to 28. The majority of occlusions was located in the middle cerebral artery (70%), with 60% in M1 and 10% in M2; 21.7% were in the internal carotid artery, with 8.3% in the vertebrobasilar artery.
Mean hours from symptom onset to arterial puncture was 3.5 ±1.4; 46.7% of patients were treated in ≤3 hours. The majority of patients (60%) received intravenous tissue plasminogen activator (tPA) prior to the embolectomy procedure but had a persistent occlusion. Other adjuvant intraarterial pharmacological agents included intraarterial tPA (10%), IA IIb/IIIa inhibitor (3.3%), and intraarterial vasodilator (3.3%).
Recanalization results showed that 91.7% of patients achieved a TICI score of 2a or higher. A TICI score of 2b or higher was achieved in 78.3% of patients. Intracranial hemorrhage (ICH) occurred in 30% of patients versus sICH (according to SITS-MOST criteria) in 5%, with 1 device-related perforation and asymptomatic ICH in 25%.
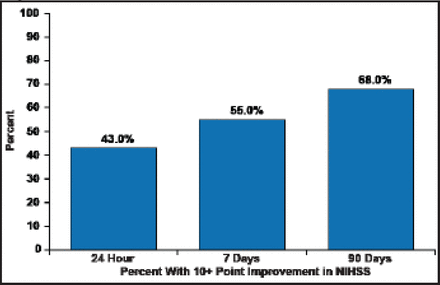
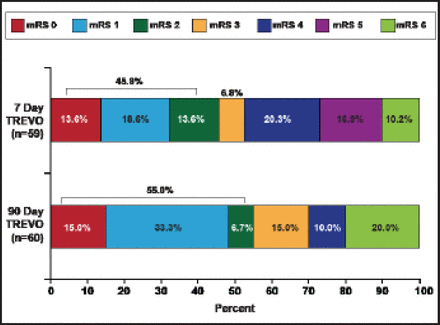
The median NIHSS decreased 47% to 9.5 in 24 hours, 75% to 4.5 at 7 days, and 89% to 2.0 in 90 days. These decreases were mirrored in clinical improvement, as defined by a 10-point improvement in NIHSS (Figure 1) and mRS outcomes (Figure 2).
Clinical Improvement in NIHSS.
Reproduced with permission from N. Wahlgren, MD, PhD.
mRS Outcomes.
Reproduced with permission from N. Wahlgren, MD, PhD.
Although TREVO was not a randomized trial, the results are very encouraging and warrant further development.
- © 2012 MD Conference Express®