Summary
There are several antiplatelet agents with diverse mechanisms of action that are or will be available for treatment of the growing population of patients with cardiovascular disease. The challenge will be to understand which patients may benefit from these therapies in combination with current treatment regimens and in the context of increasingly complex patients (eg, the elderly and diabetics).
- Thrombotic Disorders
- Coronary Artery Disease
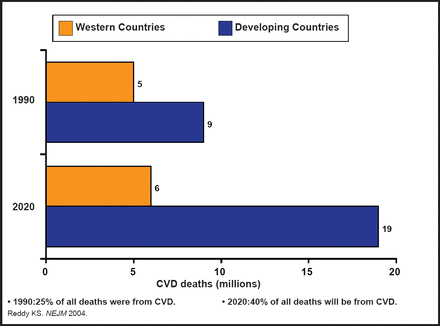
There are several antiplatelet agents with diverse mechanisms of action that are or will be available for treatment of the growing population of patients with cardiovascular disease (CVD; Figure 1). The challenge, according to Robert A. Harrington, MD, Duke University, Durham, North Carolina, USA, will be to understand which patients may benefit from these therapies in combination with current treatment regimens and in the context of increasingly complex patients (eg, the elderly and diabetics). Although it is generally agreed that a personalized approach to therapy that is based on genetics and the phenotype of response is the future for selecting platelet therapy, “we are not there yet,” said Dr. Harrington.
Global Burden of CVD.
Since thrombosis is a key component in the pathobiology of acute CVD and because adenosine diphosphate (ADP) signaling through P2Y1 and P2Y12 contributes to platelet adhesion and activation [Massucato M et al. Blood 2004], effective therapy includes blockage of the ADP receptor. Thus, ADP receptors have become the focus of much of the current research.
Elinogrel is a direct-acting, reversible P2Y12 receptor antagonist with low potential for drug-drug interactions that produces immediate and potent inhibition of ADP-mediated platelet activation when administered intravenously (IV). This agent is also active in oral form, creating the potential for flexible dosing and transition from IV to oral administration. Data from the INNOVATE-PCI Phase 2 study (NCT00751231) showed that in patients who were undergoing nonurgent percutaneous coronary intervention (PCI), elinogrel provided significantly greater (p<0.025) and faster inhibition of platelet aggregation than clopidogrel in response to platelet activation with 5 μM ADP.
Cangrelor is a direct-acting platelet P2Y12 receptor antagonist that produces rapid platelet inhibition (>90%) at 4 mg/kg/min and has a short half-life (t1/2=3 to 5 minutes). Full recovery of platelet function occurs in <60 minutes. Disappointing interim results led to the abandonment of the two CHAMPION clinical trials (NCT00305162, NCT001156571) in mid-2009 [Harrington RA et al. New Engl J Med 2009; Bhatt DJ et al. New Engl J Med 2009]. However, the BRIDGE study (NCT00767507), for short-term use prior to coronary artery bypass grafting, continues and is expected to complete in June 2013.
Vorapaxar is a protease-activated receptor 1 (PAR-1) antagonist that blocks thrombin-mediated platelet activation. In a Phase 2 study in patients who were undergoing nonurgent PCI or coronary angiography with planned PCI, oral vorapaxar did not cause an increase in TIMI bleeding, even when administered concomitantly with aspirin and clopidogrel [Becker RC et al. Lancet 2009]; however, the more recent TRACER trial (NCT00527943), investigating the use of vorapaxar in ACS, was recently stopped early due to excessive bleeding. The TRA-2P TIMI-50 study (NCT00526474), which is investigating vorapaxar across a broad range of stable atherosclerotic diseases, is continuing in patients with a history of myocardial infarction and peripheral vascular disease. Patients who enrolled with a history of ischemic stroke or who developed a stroke after randomization have been stopped prematurely, based on the Data Safety Monitoring Board findings in TRACER [Merck Statement on Changes to Clinical Studies of Vorapaxar January 13, 2011. Press Release].
The development of potent antiplatelet agents that work through diverse mechanisms raises the concern for bleeding. Aptamers (protein-binding oligonucleotides) are reversible antagonists of coagulation factor IXa. Oligonucleotides that are complementary to these aptamers can act as antidotes to their corresponding partners and are capable of restoring platelet function quickly and effectively over a clinically relevant period, thus opening the way for the development of safer, antidote-controlled platelet inhibitors [Rusconi CP et al. Nature 2002]. Clinical studies with such aptamers are eagerly awaited.
- © 2011 MD Conference Express