Summary
The 2-year results from the Endovascular Valve Edge-to-Edge Repair trial [EVEREST II; NCT00209274] showed that percutaneous mitral valve repair is safe and durable with measurable clinical benefits and is a therapeutic option for select patients with significant mitral regurgitation [Feldman T et al. N Engl J Med 2011].
- Valvular Disease
- Interventional Techniques & Devices Clinical Trials
Ted Feldman, MD, North Shore University Health System, Evanston, Illinois, USA, reported the 2-year results from the Endovascular Valve Edge-to-Edge Repair trial (EVEREST; NCT00209274), showing that percutaneous mitral valve (MV) repair is safe and durable with measurable clinical benefits and is a therapeutic option for select patients with significant mitral regurgitation (MR) [Feldman T et al. New Engl J Med 2011].
The EVEREST trial comprised patients with moderate/severe (3+) or severe (4+) MR who were candidates for MV surgery and compared percutaneous MV repair using the MitraClip device with MV surgery. The primary composite endpoint was freedom from death, surgery for mitral valve dysfunction, and grade 3+ or 4+ MR at 12 months, using an intention-to-treat (ITT) analysis. The primary safety endpoint was a composite of major adverse events within 30 days.
A total of 279 patients were randomly assigned in a 2:1 ratio to percutaneous repair (n=184) or surgery (n=95). At 2 years, 12 patients in the percutaneous arm (7%) and 12 patients in the surgical arm (12%) had missing data. Patients were well matched in terms of age and comorbidities, with the exception of history of congestive heart failure, which was more frequent in the percutaneous arm (91% vs 78%; p=0.005). About three-fourths of subjects had degenerative MR, and 27% had functional etiology. Ejection fraction was well preserved in both groups.
The primary results of the trial showed significantly higher rates of freedom from death, surgery for MV dysfunction, or grade 3+ or 4+ MR at 12 months in those who were randomized to surgery (73%) versus 55% in the percutaneous arm (p=0.007); however, there was no difference in death (a component of the primary endpoint; 6% in each group). Surgery also achieved a greater reduction in MR (p<0.001). When stratified by MR type, patients with degenerative MR did better with surgery, with a significantly higher rate of freedom from the primary endpoint (82% surgery vs 56% percutaneous; p for interaction=0.02). The primary safety endpoint of major adverse events at 30 days was significantly lower in the percutaneous arm (15% percutaneous vs 48% surgery; p<0.001).
The 2-year results showed stability in the outcomes between Year 1 and Year 2. In the 2-year analysis, the rates of the primary composite endpoint were similar to those that were observed in Year 1 (66% surgery vs 52% percutaneous; p=0.04). In addition, the proportion of patients in the percutaneous group who remained free from MV surgery at Year 2 (78.2%) was similar to that at Year 1 (78.8%). There was no difference in mortality between groups at 2 years (11%). MR grade remained stable in both groups, with the more favorable reduction in MR observed in the surgical group at Year 1, persisting through Year 2 (Table 1). Interestingly, NYHA functional class showed a more favorable outcome at both times for the percutaneous group. Importantly, there were no events of device embolization, fracture, erosion, or migration that were reported, and there was no additional occurrence of single leaflet device attachment between 1 and 2 years (6.3% at 1 year).
LV Volumes: Intention-to-Treat.
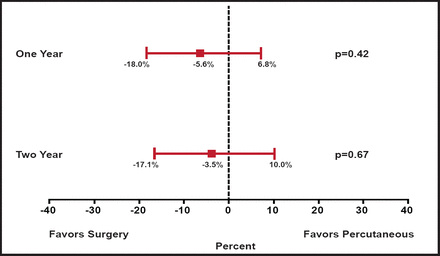
While the primary ITT analysis favored surgery and counted subsequent MV surgery following percutaneous repair as an “endpoint” event, a second analysis that evaluated the percutaneous strategy was also presented, in which subsequent MV surgery within 90 days of the percutaneous procedure was not considered an endpoint. In this secondary analysis, the differences between treatments were no longer significant (63% percutaneous vs 66% surgery; p=0.67; Figure 1). The presenter observed that “the need for surgery in patients in the clip group was almost entirely in the first several months after therapy, and after 6 months the curves overlapped at 1 and 2 years.”
Primary Effectiveness Analysis at 1 and 2 Years.
Reproduced with permission from T. Feldman, MD.
The Year 1 results of this trial showed that percutaneous repair was less effective at reducing MR than conventional surgery but that the procedure was associated with superior safety and similar improvements in clinical outcomes. The Year 2 results demonstrate overall stability in outcomes over the second year of follow-up and are reassuring, in that no device failures were observed over this period. Longer-term follow-up information will be helpful in assessing the durability of catheter-based MV repair.
- © 2011 MD Conference Express