Summary
During a joint session of the American Heart Association and European Society of Cardiology, the biology and genetics of atrial fibrillation as the basis for understanding and managing the condition were discussed.
- Arrhythmias Genomics
Biology, Genetics, and Management of AF
At a joint session of the AHA and ESC, Stanley Nattel, MD, Montreal Heart Institute, Montreal, Canada, discussed the biology and genetics of atrial fibrillation (AF) as the basis for understanding and managing the condition.
AF can occur due to multiple underlying factors, including heart disease, disturbances in extrinsic regulation, and genetic abnormalities. Development of AF requires an ectopic trigger activity and reentrant substrates to permit the maintenance of the arrhythmia. In many (probably most) cases, the occurrence and manifestations of AF are determined by multiple contributors that act in concert.
Chronic coronary artery disease (CAD) is a significant risk factor for AF, increasing the risk 3-fold postmyocardial infarction [Krahn AD et al. Am J Med 1995]. Nishida et al. recently determined the effects of chronic ischemia/infarction on AF-related substrates in an animal model, providing novel insights into potential underlying mechanisms of AF in patients with CAD [Nishida K et al. Circulation 2011].
Wang et al. identified the Pitx2 homeobox gene (a gene that contains a short DNA sequence), which is involved in the development of the myocardial sleeve around pulmonary veins, as a key player in many forms of AF [Wang J et al. Proc Natl Acad Sci USA 2010]. In another study, Body et al. found that noncoding single-nucleotide polymorphisms (SNPs) within the chromosome 4q25 region are independently associated with postoperative AF after coronary artery bypass grafting [Body SC et al. Circ Cardiovasc Genet 2009].
Whether these new clinical and genetic discoveries will translate into meaningful changes in clinicians' ability to predict which patients will develop AF or which patients will respond to prevention or active therapies remains unknown but is actively being pursued.
Ablation of AF – How, When, and What Else?
Ablation is the only proven treatment that is capable of eliminating AF in a substantial proportion of patients. Hakan Oral, MD, University of Michigan, Ann Arbor, Michigan, USA, discussed how and when catheter ablation should be initiated for the treatment of AF.
Targeting the underlying mechanisms is imperative for successful treatment with catheter ablation. These include addressing the arrhythmogenicity of pulmonary and other thoracic veins; autonomic dysregulation; fixed and/or functional reentry with fibrillatory conduction, including multiple wavelets and high frequency sources (eg, rotors); and electroanatomical remodeling [HRS/EHRA/ECAS Expert Consensus Statement. Heart Rhythm 2007].
Pulmonary veins have a dominant role in AF [Haissaguerre M et al. N Engl J Med 1998]. Therefore, ablation strategies that target the pulmonary veins and/or the antrum with the goal of complete electrical isolation are the cornerstone for most AF procedures. For surgical pulmonary vein isolation, entrance and/or exit blocks should be demonstrated [Heart Rhythm Society Guidelines. Heart Rhythm 2007].
Patients with AF of longer duration, such as those with persistent or permanent AF, may require targeting of additional sites (eg, left atrium, coronary sinus, superior vena cava, ganglionated plexi, and complex fractionated atrial electrograms). Data show that use of this tailored approach eliminated paroxysmal AF in approximately 80% of patients [Oral H et al. Circulation 2006]. Despite these advances, the long-term efficacy of catheter ablation to prevent recurrent AF requires further study. The totality of data suggests that AF ablation achieves ≥1 year of freedom from recurrent AF in many patients who are carefully selected to undergo the procedure. However, patients can have recurrent asymptomatic or symptomatic and unrecognized AF at any time after an ablation procedure. Therefore, adherence to anticoagulation is strictly required—in particular, in those with multiple CHADS2 risk factors for stroke that is associated with AF. Ongoing research in patients may reveal more about the long-term success of AF ablation with heart failure and other advanced structural heart disease.
Bottom Line for Referring Clinicians to their EP Colleagues
Indications for catheter AF ablation include symptomatic AF that is refractory or intolerant to at least one class I or III antiarrhythmic medication. AF ablation may be performed in selected symptomatic patients with heart failure and/or reduced EF. In rare clinical situations, it may be appropriate as first-line therapy. However, even after a successful AF ablation, it is important to recognize that the ACC/AHA/ESC 2011 guideline recommendations for anticoagulation at the time of cardioversion apply to patients who are in AF at the time of AF ablation (as AF termination is the goal of the procedure), including recommendations for the duration of therapeutic anticoagulation before and after the procedure. The presence of an LA thrombus, even if a patient is on a therapeutic anticoagulant, is a contraindication for catheter ablation of AF [HRS/EHRA/ECAS Consensus Statement. Heart Rhythm 2007]. The efficacy of the novel oral direct IIa and Xa anticoagulants that are rapidly being approved for use in routine clinical practice in terms of use around the time of AF ablation and cardioversion procedures is actively being explored but not definitively known. In patients with a CHADS2 risk score of 2 or higher, anticoagulation should be continued indefinitely, even after successful ablation.
ACCF/AHA/HRS Guideline Recommendation for Duration of Anticoagulation after AF Ablation (2011 ACCF/AHA/HRS Focused Update on the Management of Patients with AF [Updating the 2006 Guideline])
-
Therapeutic warfarin (INR goal: 2–3) is recommended for all patients for at least 2 months following an AF ablation.
-
Low-molecular-weight heparin or IV heparin should be used as a bridge to resumption of systemic anticoagulation following AF ablation.
-
Decisions regarding the use of warfarin more than 2 months following ablation should be based on the patient's risk factors for stroke that is associated with AF and not on the presence or type of AF. Risk determination can be aided by the CHADS2 and CHA2DS2-Vasc scores.
-
Discontinuation of warfarin therapy postablation is generally not recommended in patients who have a CHADS2 score ≥ 2.
Anticoagulation for AF
Elaine M. Hylek, MD, MPH, Boston University Medical Center, Boston, Massachusetts, USA, discussed anticoagulation for AF.
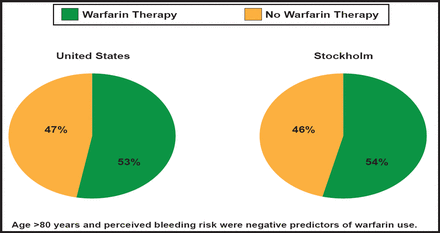
According to Dr. Hylek, five randomized trials in nonrheumatic AF (AFASAK, SPAF, BAATAF, CAFA, and SPINAF) found significant efficacy in the use of warfarin—a 66% overall risk reduction for stroke—however, only about half of high-risk patients with AF receive warfarin therapy in routine clinical practice (Figure 1) [Friberg L et al. Eur Heart J 2006; Waldo AL et al. J Am Coll Cardiol 2005].
Underutilization of Anticoagulation Therapy in AF.
Reproduced with permission from E. Hylek, MD.
Novel anticoagulants for stroke prevention in AF include dabigatran (RE-LY), apixaban (ARISTOTLE), rivaroxaban (ROCKET-AF), and edoxaban (ENGAGE AF-TIMI 48; NCT00781391) [Connolly SJ et al. N Engl J Med 2009; Granger CB et al; N Engl J Med 2011; Patel M et al. N Engl J Med 2011; Ruff CT Am Heart J 2010]. All of these trials have been completed, except ENGAGE (estimated end date in 2012).
Based on research findings from the pivotal Phase 3 studies, the United States Food and Drug Administration (FDA) approved dabigatran and rivaroxaban as having similar efficacy as therapeutic warfarin to reduce the risk of stroke in patients with nonvalvular AF. The FDA granted a priority review to apixaban in March 2012 for the prevention of stroke and systemic embolism in AF patients, given the positive results in ARISTOTLE that demonstrated reduction in stroke, bleeding, and mortality. ENGAGE AF-TIMI 48 is slated to end in 2012 and may provide for more flexible dosing (two doses studied, dose adjustments made before and after randomization). However, further research is needed to understand how to optimize the effectiveness of these novel agents in routine practice.
- © 2011 MD Conference Express®